481406
NF-κB Activation Inhibitor
The NF-κB Activation Inhibitor, also referenced under CAS 545380-34-5, controls the biological activity of NF-κB. This small molecule/inhibitor is primarily used for Inflammation/Immunology applications.
同義詞:
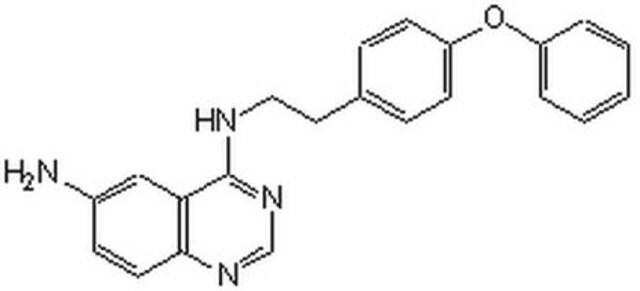
NF-κB Activation Inhibitor, 6-Amino-4-(4-phenoxyphenylethylamino)quinazoline
登入查看組織和合約定價
全部照片(2)
About This Item
推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
off-white
溶解度
DMSO: 5 mg/mL
運輸包裝
ambient
儲存溫度
2-8°C
InChI
1S/C22H20N4O/c23-17-8-11-21-20(14-17)22(26-15-25-21)24-13-12-16-6-9-19(10-7-16)27-18-4-2-1-3-5-18/h1-11,14-15H,12-13,23H2,(H,24,25,26)
InChI 密鑰
IBAKVEUZKHOWNG-UHFFFAOYSA-N
一般說明
A cell-permeable quinazoline compound that acts as a highly potent inhibitor of NF-κB transcriptional activation (IC50 = 11 nM in Jurkat cells) and LPS-induced TNF-α production (IC50 = 7 nM in murine splenocytes). Shown to exhibit anti-inflammatory effect on carrageenin-induced paw edema in rat. A 10 mM (1 mg/281 µl) solution of NF-κB Activation Inhibitor (Cat. No. 481407) in DMSO is also available.
A cell-permeable quinazoline compound that acts as a potent inhibitor of NF-κB transcriptional activation (IC50 = 11 nM in Jurkat cells) and LPS-induced TNF-α production (IC50 = 7 nM in murine splenocytes). Does not exhibit cellular cytotoxicity even at concentrations as high as 10 µM. Reported to exhibit anti-inflammatory effects on carregeenin-induced paw edema in rats.
生化/生理作用
Cell permeable: yes
Primary Target
NF-κB transcriptional activation
NF-κB transcriptional activation
Product does not compete with ATP.
Reversible: no
Target IC50: 11 nM against NF-κB transcriptional activation in Jurkat cells
包裝
Packaged under inert gas
警告
Toxicity: Toxic (F)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
其他說明
Due to the nature of the Hazardous Materials in this shipment, additional shipping charges may be applied to your order. Certain sizes may be exempt from the additional hazardous materials shipping charges. Please contact your local sales office for more information regarding these charges.
Tobe, M., et al. 2003. Bioorg. Med. Chem.11, 383.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Seongjae Kim et al.
Journal of microbiology and biotechnology, 27(10), 1820-1826 (2017-08-03)
Lipoteichoic acid (LTA), a cell wall component of gram-positive bacteria, is recognized by Toll-like receptor 2, expressed on certain mammalian cell surfaces, initiating signaling cascades that include nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase. There
Pascal Baret et al.
BioFactors (Oxford, England), 43(4), 577-592 (2017-05-26)
Diabetes and obesity are strongly associated with increased levels of circulating advanced glycation end products (AGEs) and reactive oxygen species (ROS). These two molecular phenomena affect the physiology of adipose tissue, a biological driver of the metabolic syndrome, leading to
Veronica De Paolis et al.
International journal of molecular sciences, 23(14) (2022-07-28)
The cellular heterogeneity of the tumor environment of breast cancer (BC) is extremely complex and includes different actors such as neoplastic, stromal, and immunosuppressive cells, which contribute to the chemical and mechanical modification of the environment surrounding the tumor-exasperating immune-escaping
Sara Beji et al.
BMC biology, 19(1), 124-124 (2021-06-18)
Doxorubicin (Dox) is an anti-cancer anthracycline drug that causes double-stranded DNA breaks. It is highly effective against several types of tumours; however, it also has adverse effects on regenerative populations of normal cells, such as human cardiac mesenchymal progenitor cells
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務