全部照片(2)
About This Item
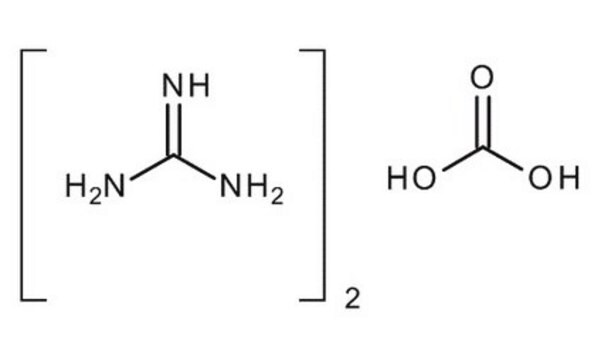
線性公式:
NH2C(=NH)NH2 · HCl
CAS號碼:
分子量::
95.53
Beilstein:
3591990
MDL號碼:
分類程式碼代碼:
12352107
酶委員會索引編號:
200-002-3
推薦產品
agency
NF
品質等級
產品線
EMPROVE® EXPERT
效力
655.3-907.1 mg/kg LD50, oral (Rat)
>2000 mg/kg LD50, skin (Rabbit)
特點
RNase and DNase free
pH值
4.5-5.5 (20 °C, 100 g/L in H2O)
mp
180-185 °C (lit.)
188 °C
溶解度
2150 g/L
密度
1.3 g/cm3 (lit.)
體積密度
550‑620 kg/m3
儲存溫度
15-25°C
SMILES 字串
Cl[H].NC(N)=N
InChI
1S/CH5N3.ClH/c2-1(3)4;/h(H5,2,3,4);1H
InChI 密鑰
PJJJBBJSCAKJQF-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Finding the right chemical that matches your pharma and biopharma manufacturing needs as well as regulatory demands can be a complicated challenge. With our application know-how and regulatory expertise, we support you in every step of development, scale-up, and production.As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for pharma and biopharma manufacturing withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
Our SAFC® portfolio of high-quality products for biopharmaceutical processing withstands strict quality control procedures and is produced according to MQ-500 requirements as defined by the M-Clarity program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of downstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Ranging from non-GMP grades for low-risk application, to IPEC-PQG GMP for higher-risk applications, we have products covering all your manufacturing needs.
應用
强效离液剂,用于蛋白质的变性以及后续复性。该强效变性剂可溶解不溶性或变性的蛋白质,如包涵体。可用作使蛋白质或酶复性为活性形式的第一步骤。可能还需要脲和二硫苏糖醇 (DTT)。
其他說明
Due to its low microbial and endotoxin limits, Guanidinium chloride Emprove® Expert is suitable for biopharma manufacturing needs.
法律資訊
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFC is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Warning
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務