推薦產品
形狀
powder
包裝
pkg of 1 × 1 mg (860665P-1mg)
製造商/商標名
Avanti Research™ - A Croda Brand 860665P
脂質類型
sphingolipids
運輸包裝
dry ice
儲存溫度
−20°C
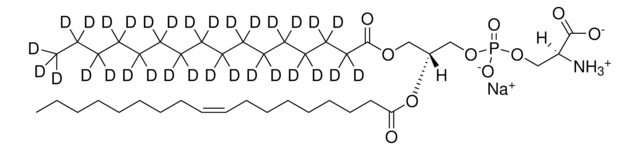
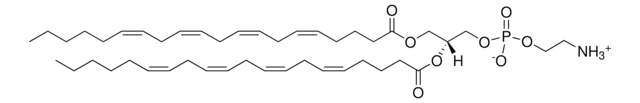
SMILES 字串
OC[C@@](N)([H])[C@]([H])(O)/C=C/CCCCCCCC/C=C\CCC
InChI
1S/C18H35NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h4-5,14-15,17-18,20-21H,2-3,6-13,16,19H2,1H3/b5-4-,15-14+
InChI 密鑰
KWDXKYNWAKMLKK-YQMRQDNGSA-N
一般說明
4E,14Z-Sphingadiene is a unique sphingoid base containing C18 chain, with trans double bond at C-4 and cis double bond between C14 and C15 with a bent structure. It is found in the plasma, lymph, brain, kidney and lungs of humans and mice. 4E,14Z-Sphingadiene or d-erythro-1,3-dihydroxy-2-amino-4-trans-14-cis-octadecadiene is the second largest long-chain base of human plasma sphingomyelins.
生化/生理作用
Sphingadiene, a vital component of sphingolipids facilitates numerous processes such as apoptosis and cell signaling. It inhibits Akt/Wnt signaling and acts as an anticancer agent in colon cancer.
包裝
5 mL Amber Glass Screw Cap Vial (860665P-1mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
O Renkonen et al.
Journal of lipid research, 10(6), 687-693 (1969-11-01)
The dienic long-chain base (sphingadienine) of human plasma sphingomyelins has been identified as d-erythro-1,3-dihydroxy-2-amino-4-trans-14-cis-octadecadiene. A similar sphingosine was also detected in plasma sphingomyelins of rat, rabbit, and cat. The key reaction in the structural studies was partial reduction of sphingadienine
Occurrence, structure elucidation, biosynthesis, functions and synthesis of sphingadienes
U Abeytunga T
Mini-Reviews in Organic Chemistry, 12(3), 282-292 (2015)
Keisuke Jojima et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 34(2), 3318-3335 (2020-01-10)
Sphingolipids are multifunctional lipids. Among the sphingolipid-component sphingoid bases, 4,14-sphingadiene (SPD) is unique such that it has a cis double bond with a bent structure. Although SPD was discovered half a century ago, its tissue distribution, biosynthesis, and degradation remain
Sarah T Pruett et al.
Journal of lipid research, 49(8), 1621-1639 (2008-05-24)
"Sphingosin" was first described by J. L. W. Thudichum in 1884 and structurally characterized as 2S,3R,4E-2-aminooctadec-4-ene-1,3-diol in 1947 by Herb Carter, who also proposed the designation of "lipides derived from sphingosine as sphingolipides." This category of amino alcohols is now
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務