推薦產品
形狀
powder
包裝
pkg of 1 × 10 mg (860654P-10mg)
pkg of 1 × 5 mg (860654P-5mg)
製造商/商標名
Avanti Research™ - A Croda Brand 860654P
脂質類型
sphingolipids
運輸包裝
dry ice
儲存溫度
−20°C
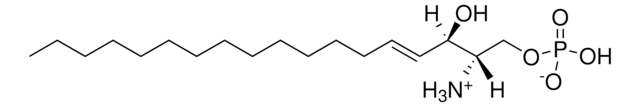
SMILES 字串
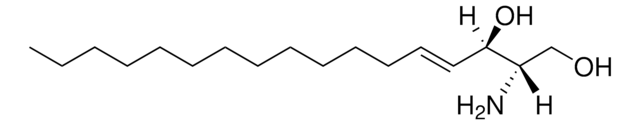
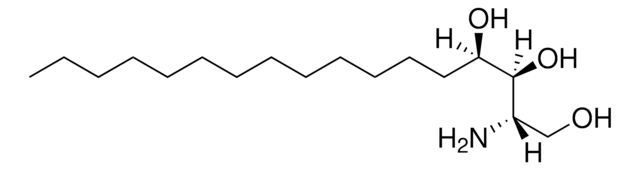
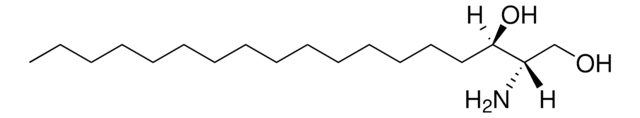
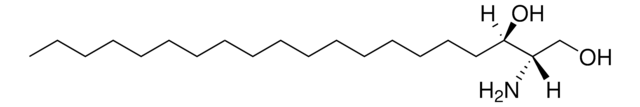
OC[C@@](N)([H])[C@]([H])(O)CCCCCCCCCCCCCC
InChI
1S/C17H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-17(20)16(18)15-19/h16-17,19-20H,2-15,18H2,1H3/t16-,17+/m0/s1
InChI 密鑰
KFQUQCFJDMSIJF-DLBZAZTESA-N
一般說明
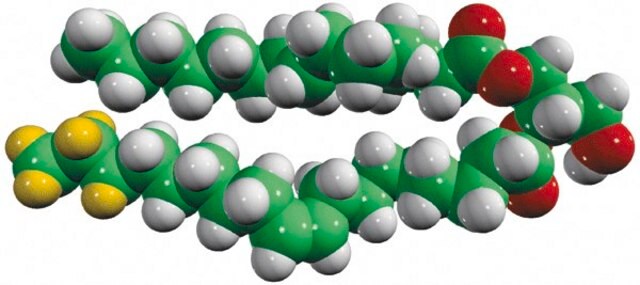
Sphinganine (d17:0), also referred to as dihydrosphingosine, is devoid of double bond. Decarboxylating condensation of serine with palmitoyl-CoA yield keto intermediate(2-oxosphinganine), which is then reduced by NADPH to form Sphinganine.
應用
Sphinganine (d17:0) has been used in liquid chromatography-mass spectrometry (LC-MS) for the in-situ estimation of dihydrosphingosine utilization in N-(4-hydroxyphenyl)retinamide (4-HPR) resistant leukemia cells. It is suitable for use as an internal standard in metabolomic study.
生化/生理作用
Sphinganine (SPA) or D-erythro-sphinganine is involved in sphingolipid biosynthesis pathway. It acts as a precursor for ceramide. SPA has an ability to inhibit the activity of protein kinase c. SPA accumulation enables fumonisin B1 to stimulate apoptosis. It also has an ability to block cholesterol transport in Niemann-Pick Type C (NPC) disease.
包裝
5 mL Amber Glass Screw Cap Vial (860654P-10mg)
5 mL Amber Glass Screw Cap Vial (860654P-5mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
也與該產品經常一起購買
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
客戶也查看了
Lipids
Basic Neurochemistry: Molecular, Cellular and Medical Aspects., 14(5), 833-876 (2011)
Quantification of sphingosine and sphinganine from crude lipid extracts by HPLC electrospray ionization tandem mass spectrometry
Lieser B, et al.
Journal of Lipid Research, 44(11), 2209-2216 (2003)
C F Roff et al.
Developmental neuroscience, 13(4-5), 315-319 (1991-01-01)
Niemann-Pick Type C (NPC) disease is a cholesterol lipidosis resulting from defective postlysosomal cholesterol transport. In normal cells this segment of cholesterol trafficking is inhibited by treatment with either U18666A or imipramine. Other compounds are also capable of blocking postlysosomal
mTORC2 promotes tumorigenesis via lipid synthesis
Guri Y, et al.
Cancer Cell, 32(6), 807-823 (2017)
Evaluation of bioactive sphingolipids in 4-HPR-resistant leukemia cells
Apraiz A, et al.
BMC Cancer, 11(1), 477-477 (2011)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務