推薦產品
化驗
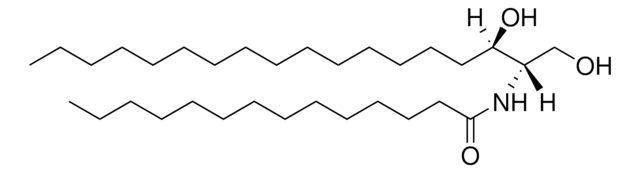
>99% (TLC)
形狀
powder
包裝
pkg of 1 × 1 mg (810222P-1MG)
pkg of 1 × 250 μg (810222P-250ug)
製造商/商標名
Avanti Research™ - A Croda Brand 810222P
運輸包裝
dry ice
儲存溫度
−20°C
生化/生理作用
The synthesis of glucosyl ceramide (GlcCer) occurs in the cytosolic membranes of Golgi. It is catalysed by GlcCer synthase. In the luminal Golgi membrane, GlcCer participates in the generation of glycosphingolipids. C6-NBD glcCer is useful to view ceramide rich membrane domains in Golgi compartments.
包裝
5 mL Amber Glass Screw Cap Vial (810299P-1MG)
5 mL Clear Glass Sealed Ampule (810222P-250ug)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
No data available
閃點(°C)
No data available
A functionalized sphingolipid analogue for studying redistribution during activation in living T cells
Collenburg L, et al.
Journal of Immunology, 196(9), 3951-3962 (2016)
Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide
D?Angelo G, et al.
Nature, 449(7158), 62-62 (2007)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務