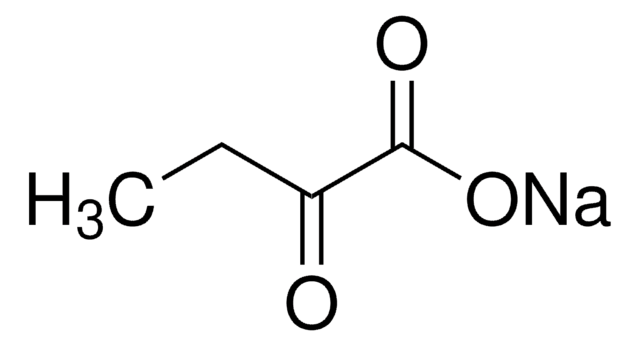
推薦產品
生物源
synthetic
品質等級
等級
FG
Fragrance grade
Kosher
agency
follows IFRA guidelines
法律遵循
EU Regulation 1223/2009
EU Regulation 1334/2008 & 872/2012
化驗
≥95%
bp
84 °C/20 mmHg (lit.)
mp
30-34 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
香料過敏原
no known allergens
感官的
caramel; creamy; brown; sweet
儲存溫度
2-8°C
SMILES 字串
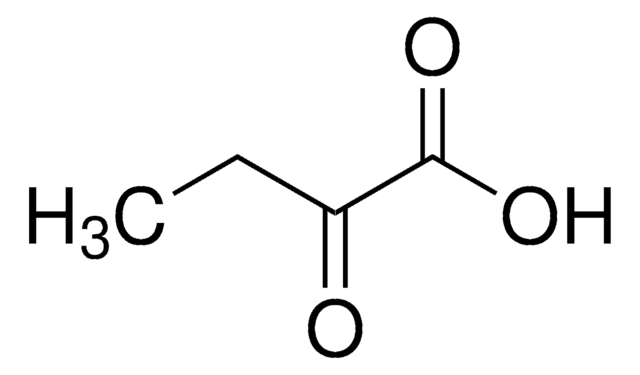
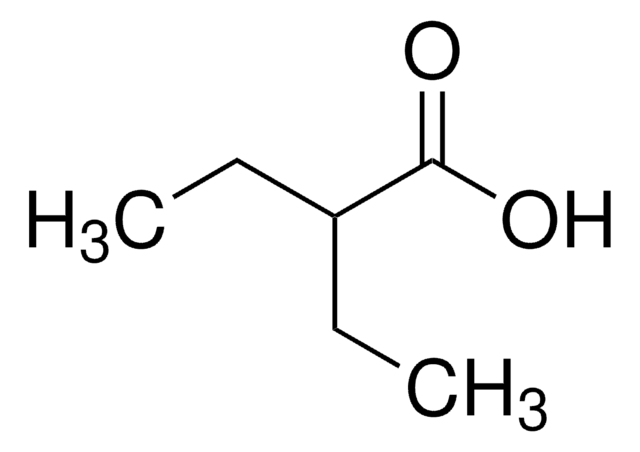
CCC(=O)C(O)=O
InChI
1S/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7)
InChI 密鑰
TYEYBOSBBBHJIV-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
2-Oxobutyric acid is mainly found in the hydrolysates of proteins, reportedly being formed by the degradation of threonine.
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
179.6 °F - closed cup
閃點(°C)
82 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Review of thermally produced imitation meat flavors.
Wilson RA
Journal of Agricultural and Food Chemistry, 23(6), 1032-1037 (1975)
A Probable Flavoring Principle in Vegetable?Protein Hydrolysates.
Sulser H, et al.
Journal of Food Science, 32(6), 611-615 (1967)
K Yaegaki et al.
Journal of periodontology, 63(9), 783-789 (1992-09-01)
The amounts of volatile sulfur compounds (VSC) and methyl mercaptan/hydrogen sulfide ratio in mouth air from patients with periodontal involvement were 8 times greater than those of control subjects. Our studies demonstrated that, in patients with periodontal disease: 1) the
Sergey V Smirnov et al.
FEMS microbiology letters, 273(1), 70-77 (2007-06-15)
A two-step enzymatic synthesis process of 4-hydroxyisoleucine is suggested. In the first step, the aldol condensation of acetaldehyde and alpha-ketobutyrate catalyzed by specific aldolase results in the formation of 4-hydroxy-3-methyl-2-keto-pentanoate (HMKP). In the second step, amination of HMKP by the
Asymmetric formal [3+2] cycloaddition reaction of isocyanoesters to 2-oxobutenoate esters by a multifunctional chiral silver catalyst.
Jin Song et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(28), 7786-7790 (2011-05-28)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務