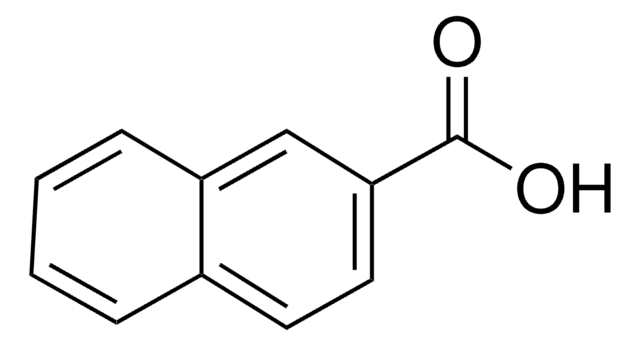
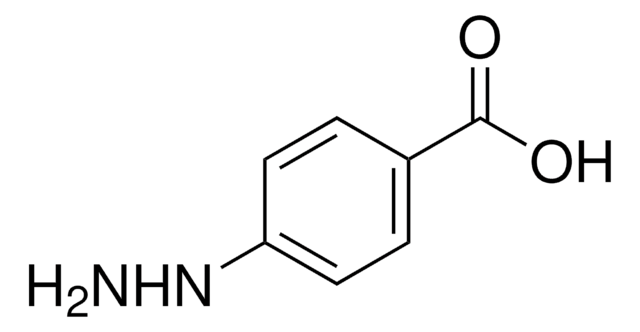
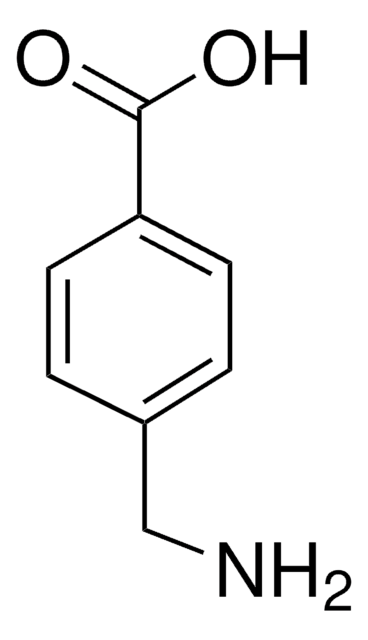
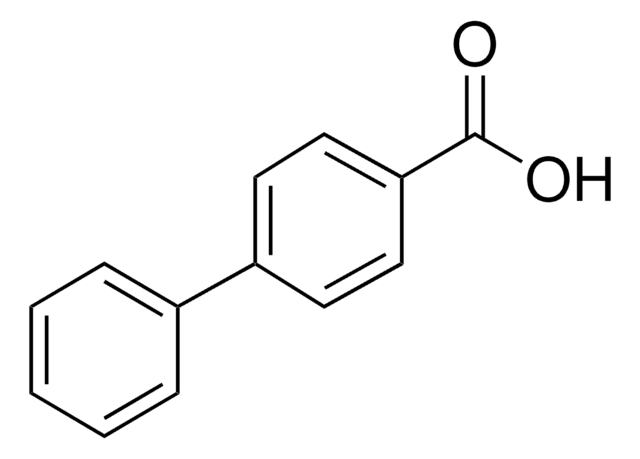
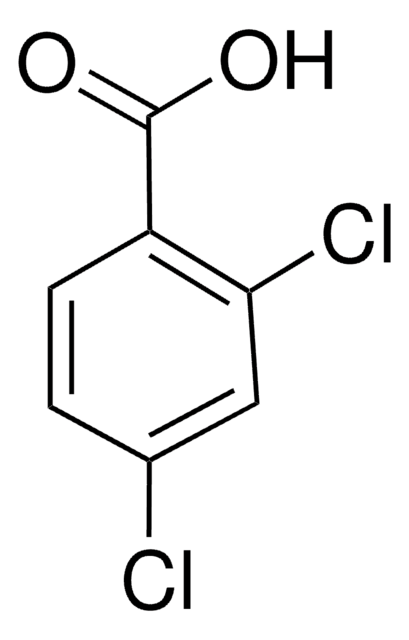
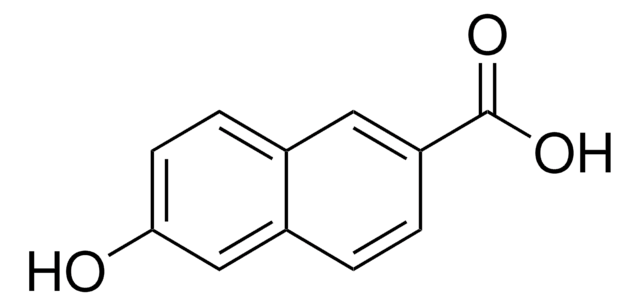
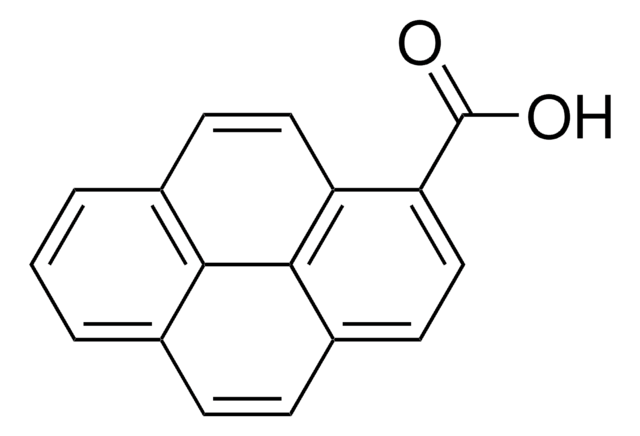
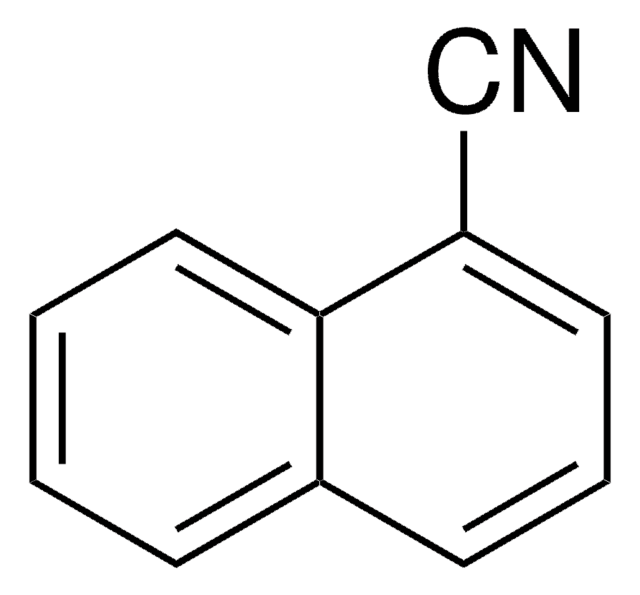
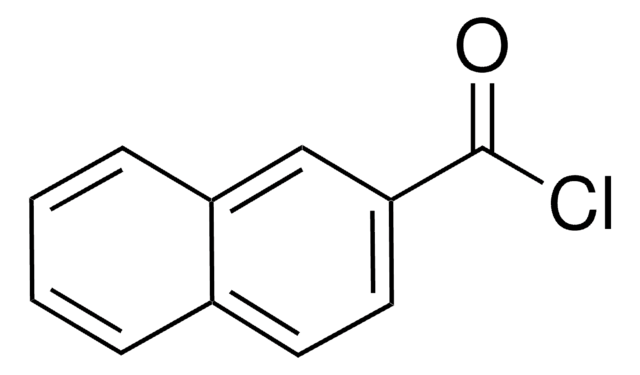
推薦產品
品質等級
化驗
96%
形狀
powder
bp
300 °C (lit.)
mp
157-160 °C (lit.)
SMILES 字串
OC(=O)c1cccc2ccccc12
InChI
1S/C11H8O2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H,(H,12,13)
InChI 密鑰
LNETULKMXZVUST-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
1-萘甲酸可作为以下应用的反应剂:
- 在铑催化剂存在下,与炔烃通过脱水环化反应制备萘嵌苯酮。
- 使用Rh催化剂,与2-丁炔通过有氧的氧化环化反应制备异香豆素衍生物。
- 与N,O-二甲基羟胺和三氯化磷反应制备N-甲氧基-N-甲基-1-萘甲酰胺(Weinreb酰胺)。
- 通过Birch还原反应制备1,4-二氢-1-萘羧酸。
其他說明
残留物为 2-萘甲酸
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Yu-mei Song et al.
Chemical communications (Cambridge, England), 48(7), 1006-1008 (2011-12-14)
Reversible single-crystal-to-single-crystal transformation (SCSC) was for the first time observed between 4f-based molecular magnets.
Hiromasa Uchiyama et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 43(1-2), 71-77 (2011-04-06)
Spray-dried particles (SDPs) with indomethacin (IND) and alpha-glycosyl transferase-treated stevia (Stevia-G) indicated extremely high dissolution rates and apparent solubility compared to particles of a ground mixture and a physical mixture of IND/Stevia-G. The apparent solubility of IND from SDPs was
Qunfei Zhao et al.
Chemistry & biology, 15(7), 693-705 (2008-07-19)
Azinomycin B is a complex natural product containing densely assembled functionalities with potent antitumor activity. Cloning and sequence analysis of the azi gene cluster revealed an iterative type I polyketide synthase (PKS) gene, five nonribosomal peptide synthetases (NRPSs) genes and
Mutual activation: Suzuki-Miyaura coupling through direct cleavage of the sp2 C-O bond of naphtholate.
Da-Gang Yu et al.
Angewandte Chemie (International ed. in English), 50(31), 7097-7100 (2011-06-29)
Rajesh Sunasee et al.
The Journal of organic chemistry, 73(20), 8016-8020 (2008-09-26)
A method is described for converting tert-butyl benzoates or tert-butyl 1-naphthoates into derivatives having an alkyl or substituted alkyl group in a 1,4-relationship to an alkyl, aryl, alkenyl, or alkynyl group. Key steps in the sequence are (i) addition of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務