推薦產品
product name
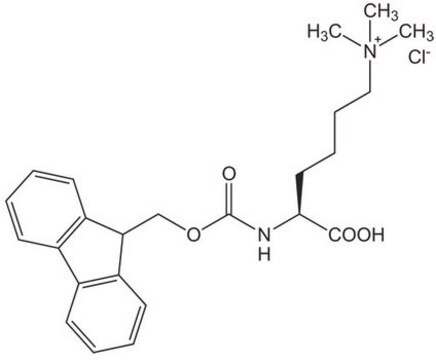
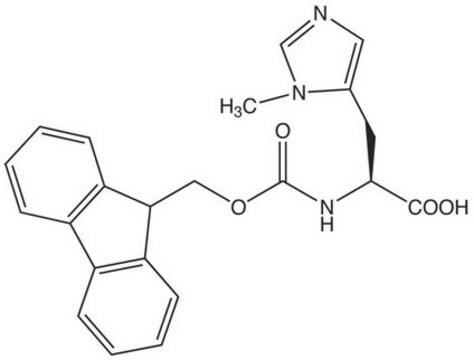
Fmoc-Lys(Me)3-OH Chloride, ≥97%
化驗
≥97%
形狀
liquid
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
應用
peptide synthesis
官能基
Fmoc
運輸包裝
dry ice
儲存溫度
−20°C
InChI
1S/C24H30N2O4.ClH/c1-26(2,3)15-9-8-14-22(23(27)28)25-24(29)30-16-21-19-12-6-4-10-17(19)18-11-5-7-13-20(18)21;/h4-7,10-13,21-22H,8-9,14-16H2,1-3H3,(H-,25,27,28,29);1H/t22-;/m0./s1
InChI 密鑰
XUJRNPVABVHOAJ-FTBISJDPSA-N
一般說明
Fmoc protected N-trimethyl lysine
應用
Fmoc-Lys(Me)3-OH chloride is one of the common N-terminal protected reagents used in the peptide synthesis. Some of the reported examples are:
- Synthesis of peptide linkers for monoclonal antibody-auristatin F conjugates.
- Preparation of multifunctional reagents with enhanced ionization properties for the analysis of protein modification in human cells and dynamic profiling of protein lipidation.
- Sequential peptide ligation to synthesize histone H3 containing a trimethyl lysine residue, with modified tail region.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Multifunctional Reagents for Quantitative Proteome?Wide Analysis of Protein Modification in Human Cells and Dynamic Profiling of Protein Lipidation During Vertebrate Development.
Broncel M, et al.
Angewandte Chemie (International Edition in English), 54(20), 5948-5951 (2015)
Novel peptide linkers for highly potent antibody? auristatin conjugate.
Doronina S O, et al.
Bioconjugate Chemistry, 19(10), 1960-1963 (2008)
Sequential Peptide Ligation by Combining the Cys?Pro Ester (CPE) and Thioester Methods and Its Application to the Synthesis of Histone H3 Containing a Trimethyl Lysine Residue.
Kawakami T, et al.
Bulletin of the Chemical Society of Japan, 86(6), 690-697 (2013)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務