推薦產品
品質等級
化驗
96%
形狀
powder
光學活性
[α]23/D −109.2°, c = 1.5 in ethanol
mp
204-206 °C (lit.)
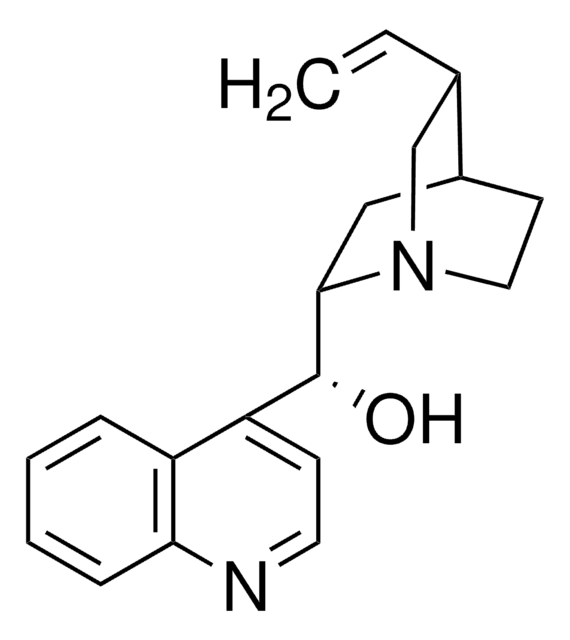
SMILES 字串
O[C@@H]([C@@H]1C[C@@H]2CCN1C[C@@H]2C=C)c3ccnc4ccccc34
InChI
1S/C19H22N2O/c1-2-13-12-21-10-8-14(13)11-18(21)19(22)16-7-9-20-17-6-4-3-5-15(16)17/h2-7,9,13-14,18-19,22H,1,8,10-12H2/t13-,14-,18-,19+/m0/s1
InChI 密鑰
KMPWYEUPVWOPIM-KODHJQJWSA-N
基因資訊
human ... CYP2D6(1565)
尋找類似的產品? 前往 產品比較指南
一般說明
Cinchonidine is a cinchona alkaloid, which belongs to the orthorhombic crystal system and P212121 space group.
應用
The enantioselective hydrogenation of ethyl pyruvate in the presence of cinchonidine modified platinum/alumina catalyst leads to the enantiomeric excess of ethyl (R)-lactate.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type N95 (US)
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
The alkaloid cinchonidine.
Oleksyn BJ
Acta Crystallographica Section B, Structural Science, Crystal Engineering and Materials, 1832-1834 (1982)
Blaser HU, et al.
Studies in Surface Science and Catalysis, 67, 147-155 (1991)
Chun-Yin Zhu et al.
Chemical communications (Cambridge, England), (6)(6), 738-740 (2008-05-16)
The reaction of cinchonidine (cinchonine)-derived ammonium salts with nitroolefins in the presence of Cs2CO3 to afford optically active isoxazoline N-oxides with excellent ee and high de values has been developed.
Arkajyoti Sengupta et al.
The Journal of organic chemistry, 77(23), 10525-10536 (2012-11-17)
Mechanism and the origin of enantioselectivity in the decarboxylative protonation of α-amino malonate hemiester promoted by epicinchona-thiourea hybrid organocatalyst is established by using the DFT(M06-2X/6-311+G**//ONIOM2) computational methods. The origin of stereoselectivity rendered by this hybrid bifunctional catalyst in asymmetric protonation
Erik Schmidt et al.
Langmuir : the ACS journal of surfaces and colloids, 23(15), 8087-8093 (2007-06-23)
Cinchona alkaloids are frequently used for chiral modification of supported noble metal catalysts employed in heterogeneous enantioselective hydrogenation. In order to gain molecular insight into the surface processes occurring at the metal/liquid interface, cinchonidine (CD) adsorption on vapor-deposited Rh/Al2O3 films
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務