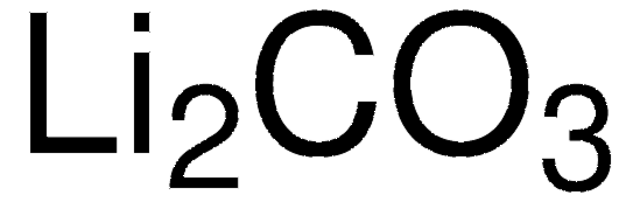推薦產品
等級
battery grade
品質等級
化驗
≥99.9% trace metals basis
環保替代產品特色
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
618 °C (lit.)
環保替代類別
SMILES 字串
[Li+].[Li+].[O-]C([O-])=O
InChI
1S/CH2O3.2Li/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2
InChI 密鑰
XGZVUEUWXADBQD-UHFFFAOYSA-L
尋找類似的產品? 前往 產品比較指南
一般說明
Lithium carbonate is a white, crystalline salt that only exists in the anhydrous form. The salt is soluble in water, but poorly, and it is insoluble in alcohols and acetone. The solubility of lithium carbonate in water decreases with increasing temperature, which is unusual for a salt. Its solubility increases with partial pressure of carbon dioxide, which drives the equilibrium towards the more soluble metastable bicarbonate. These properties of its solubility are often exploited in its purification.
Lithium carbonate is an important industrial chemical, primarily as a precursor to lithium fluoride and lithium hydroxide, key precursors for compounds used in lithium-ion batteries. It is also used directly in ceramic glazes, glasses, and fireworks, among other industrial applications.
Lithium carbonate is produced in several ways, usually involving extracting lithium from the earth. One common extraction method involves mining and acid leaching from spodumene ores (lithium aluminum silicate). The ore is concentrated, baked at high temperature to change the crystal structure to a digestible phase, then digested with sulfuric acid to form a concentrate. Reacting the lithium sulfate concentrate with sodium carbonate forms the raw lithium carbonate that is further purified and dried. Another method involves processing and purifying underwater brine, which is pumped to the surface and dried by passive evaporation. The resulting salts are converted to lithium carbonate and subsequently purified.
Lithium carbonate is an important industrial chemical, primarily as a precursor to lithium fluoride and lithium hydroxide, key precursors for compounds used in lithium-ion batteries. It is also used directly in ceramic glazes, glasses, and fireworks, among other industrial applications.
Lithium carbonate is produced in several ways, usually involving extracting lithium from the earth. One common extraction method involves mining and acid leaching from spodumene ores (lithium aluminum silicate). The ore is concentrated, baked at high temperature to change the crystal structure to a digestible phase, then digested with sulfuric acid to form a concentrate. Reacting the lithium sulfate concentrate with sodium carbonate forms the raw lithium carbonate that is further purified and dried. Another method involves processing and purifying underwater brine, which is pumped to the surface and dried by passive evaporation. The resulting salts are converted to lithium carbonate and subsequently purified.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
應用
Lithium carbonate is used in the preparation of many lithium compounds, most notably lithium iron phosphate (LiFePO4). A common synthetic strategy for synthesizing lithium metal oxides involves thermally decomposing lithium carbonate, which serves effectively as a convenient, in-situ source of lithium oxide by cleanly evolving carbon dioxide. Typically, lithium carbonate is mixed or ball-milled with other metal carbonates, metal oxides, and phosphates. Then the mixture is heated at a low temperature (e.g. 350 °C) and subsequently at a higher temperature (e.g. 600 °C) to complete the reaction and improve the crystallinity of the product. Researchers have used this technique to prepare exciting new materials for lithium-ion batteries, like Li2Ru1-ySnyO3 as a cathode material and Li7La3Zr2O12 (LLZ) as a solid-state electrolyte.
包裝
100 g in poly bottle
500 g in poly bottle
500 g in poly bottle
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention
Tsai, C., Roddatis, V., et al.
ACS Applied Materials & Interfaces, 8, 10617?10626-10617?10626 (2016)
Byoungwoo Kang et al.
Nature, 458(7235), 190-193 (2009-03-13)
The storage of electrical energy at high charge and discharge rate is an important technology in today's society, and can enable hybrid and plug-in hybrid electric vehicles and provide back-up for wind and solar energy. It is typically believed that
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務