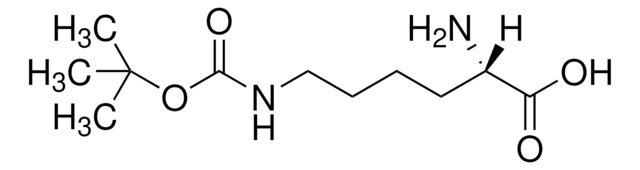
914827
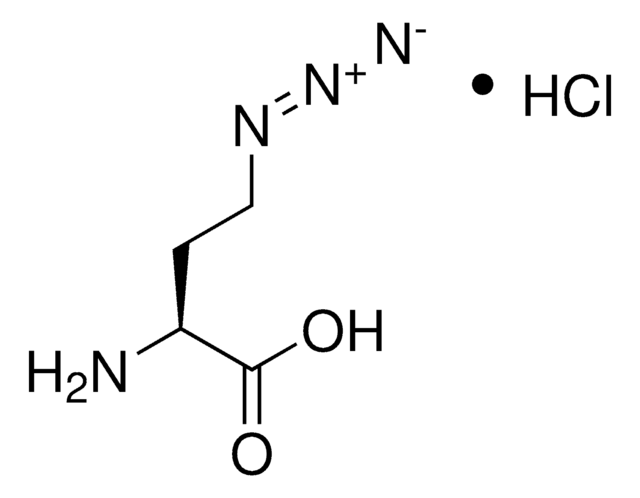
N6-((Prop-2-yn-1-yloxy)carbonyl)-L-lysine hydrochloride
≥98%
同義詞:
(S)-Amino-6-((prop-2-ynyloxy)carbonylamino)hexanoic acid hydrochloride, Clickable amino acid for bioconjugation, H-L-Lys(Poc)-OH HCl, Lysine-alkyne, Plk, Propargyl-derivatized lysize, UAA crosslinker
登入查看組織和合約定價
全部照片(2)
About This Item
推薦產品
化驗
≥98%
形狀
powder
儲存溫度
2-8°C
應用
N6-((Prop-2-yn-1-yloxy)carbonyl)-L-lysine hydrochloride is a clickable amino acid derivative for site-specific incorporation into recombinant proteins or synthesis of chemical probes and tools for biological applications. This non-canonical lysine possesses an alkyne for bioorthogonal reaction with azides.
其他說明
Site-Specific Encoding of Photoactivity in Antibodies Enables Light-Mediated Antibody-Antigen Binding on Live Cells Quick View Other Sources
PEGylated polylysine derived copolymers with reduction-responsive side chains for anticancer drug delivery
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
Combined Use of Unnatural Amino Acids Enables Dual-Color Super-Resolution Imaging of Proteins via Click Chemistry
PEGylated polylysine derived copolymers with reduction-responsive side chains for anticancer drug delivery
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
Combined Use of Unnatural Amino Acids Enables Dual-Color Super-Resolution Imaging of Proteins via Click Chemistry
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Robert Serfling et al.
Nucleic acids research, 46(1), 1-10 (2017-11-28)
The pyrrolysyl-tRNA synthetase/tRNAPyl pair is the most versatile and widespread system for the incorporation of non-canonical amino acids (ncAAs) into proteins in mammalian cells. However, low yields of ncAA incorporation severely limit its applicability to relevant biological targets. Here, we
Silvia Eger et al.
Methods in molecular biology (Clifton, N.J.), 832, 589-596 (2012-02-22)
The conjugation of poly-ubiquitin chains is a widespread post-translational modification of proteins that plays a role in many different cellular processes. Notably, the biological function of the attached ubiquitin chain depends on which lysine residue is used for chain formation.
Andreas Schmidt et al.
ACS chemical biology, 13(9), 2472-2483 (2018-08-01)
Single-molecule techniques allow unique insights into biological systems as they provide unrivaled access to structural dynamics and conformational heterogeneity. One major bottleneck for reliable single-molecule Förster resonance energy transfer (smFRET) analysis is the identification of suitable fluorophore labeling sites that
Ivana Nikić et al.
Angewandte Chemie (International ed. in English), 53(8), 2245-2249 (2014-01-30)
The growing demands of advanced fluorescence and super-resolution microscopy benefit from the development of small and highly photostable fluorescent probes. Techniques developed to expand the genetic code permit the residue-specific encoding of unnatural amino acids (UAAs) armed with novel clickable
Jihe Liu et al.
Journal of the American Chemical Society, 139(27), 9100-9103 (2017-06-29)
Site-specific incorporation of unnatural amino acids into proteins provides a powerful tool to study protein function. Here we report genetic code expansion in zebrafish embryos and its application to the optogenetic control of cell signaling. We genetically encoded four unnatural
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務


![N-[2-[2-[2-(2-叠氮乙氧基)乙氧基]乙氧基]乙基]生物素胺](/deepweb/assets/sigmaaldrich/product/structures/120/306/c9779b03-3754-4ad6-8eef-b07209e113ce/640/c9779b03-3754-4ad6-8eef-b07209e113ce.png)