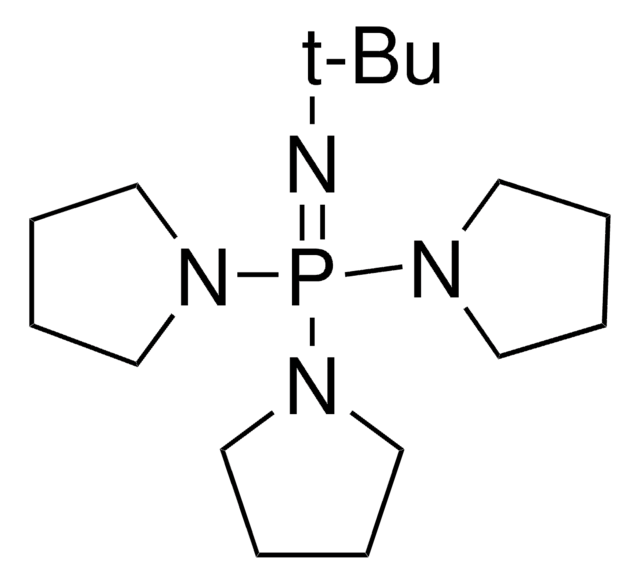
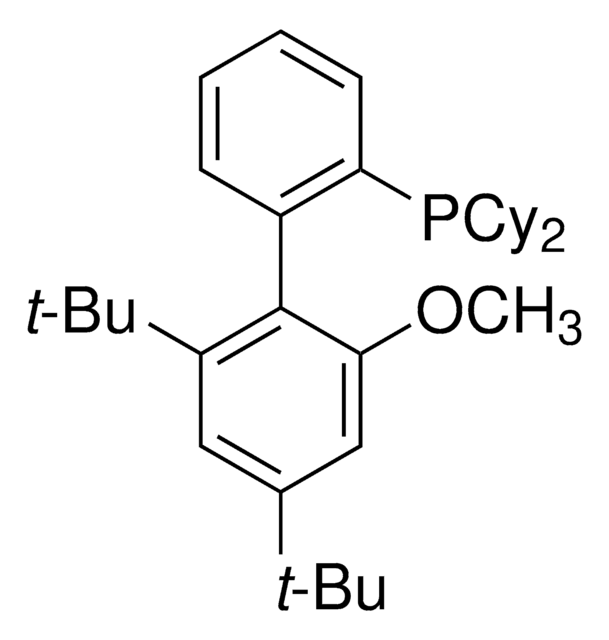
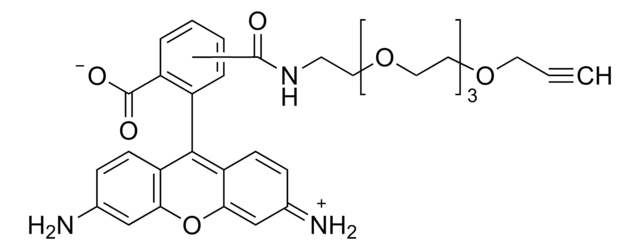
推薦產品
形狀
solid
反應適用性
reaction type: click chemistry
存貨情形
available only in USA
儲存溫度
2-8°C
SMILES 字串
[S](=O)(=O)(O)CCC[n]1nnc(c1)CN(Cc3nn[n](c3)C(C)(C)C)Cc2nn[n](c2)C(C)(C)C
InChI
1S/C20H34N10O3S/c1-19(2,3)29-14-17(22-25-29)11-27(12-18-15-30(26-23-18)20(4,5)6)10-16-13-28(24-21-16)8-7-9-34(31,32)33/h13-15H,7-12H2,1-6H3,(H,31,32,33)
InChI 密鑰
WMEZDVILBKIODK-UHFFFAOYSA-N
應用
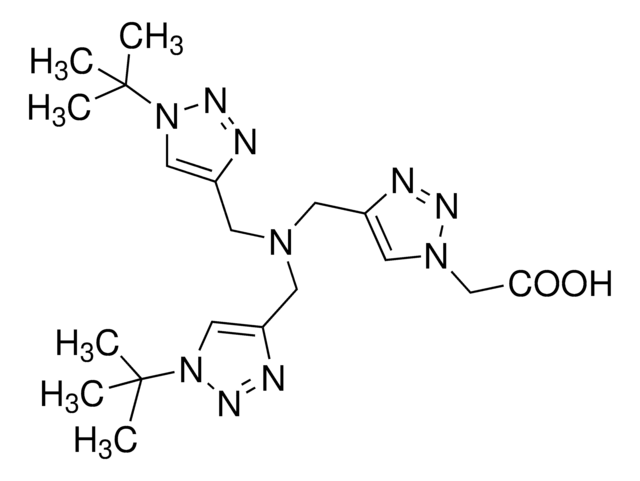
BTTES is a a next-generation, water-soluble ligand for the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) that accelerates reaction rates and suppresses cell cytotoxicity. The biocompatibility and fast kinetics of BTTES are advancements from water-insoluble TBTA and are desirable for bioconjugation in diverse chemical biology experiments.
訊號詞
Danger
危險聲明
危險分類
Self-react. C
儲存類別代碼
5.2 - Organic peroxides and self-reacting hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Wei Wang et al.
Chemistry, an Asian journal, 6(10), 2796-2802 (2011-09-10)
The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), the prototypical reaction of click chemistry, is accelerated by tris(triazolylmethyl)amine-based ligands. Herein, we compare two new ligands in this family--3-[4-({bis[(1-tert-butyl-1H-1,2,3-triazol-4-yl)methyl]amino}methyl)-1H-1,2,3-triazol-1-yl]propanol (BTTP) and the corresponding sulfated ligand 3-[4-({bis[(1-tert-butyl-1H-1,2,3-triazol-4-yl)methyl]amino}methyl)-1H-1,2,3-triazol-1-yl]propyl hydrogen sulfate (BTTPS)--for three bioconjugation applications: 1) labeling
David Soriano Del Amo et al.
Journal of the American Chemical Society, 132(47), 16893-16899 (2010-11-11)
The Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) is the standard method for bioorthogonal conjugation. However, current Cu(I) catalyst formulations are toxic, hindering their use in living systems. Here we report that BTTES, a tris(triazolylmethyl)amine-based ligand for Cu(I), promotes the cycloaddition reaction rapidly
Christen Besanceney-Webler et al.
Angewandte Chemie (International ed. in English), 50(35), 8051-8056 (2011-07-16)
Raising the bar: the efficacy of bioorthogonal reactions for bioconjugation has been thoroughly evaluated in four different biological settings. Powered by the development of new biocompatible ligands, the copper-catalyzed azide-alkyne cycloaddition has brought about unsurpassed bioconjugation efficiency, and thus it
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![三[(1-苄基-1H-1,2,3-三唑-4-基)甲基]胺 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)


![N-[2-[2-[2-(2-叠氮乙氧基)乙氧基]乙氧基]乙基]生物素胺](/deepweb/assets/sigmaaldrich/product/structures/120/306/c9779b03-3754-4ad6-8eef-b07209e113ce/640/c9779b03-3754-4ad6-8eef-b07209e113ce.png)
![四[三(二甲氨基)正膦亚基氨基]氯化磷 95%](/deepweb/assets/sigmaaldrich/product/structures/160/963/9dd6d457-17b2-44dc-8ea2-d3c0475b3664/640/9dd6d457-17b2-44dc-8ea2-d3c0475b3664.png)