推薦產品
形狀
powder or crystals
儲存溫度
−20°C
相關類別
應用
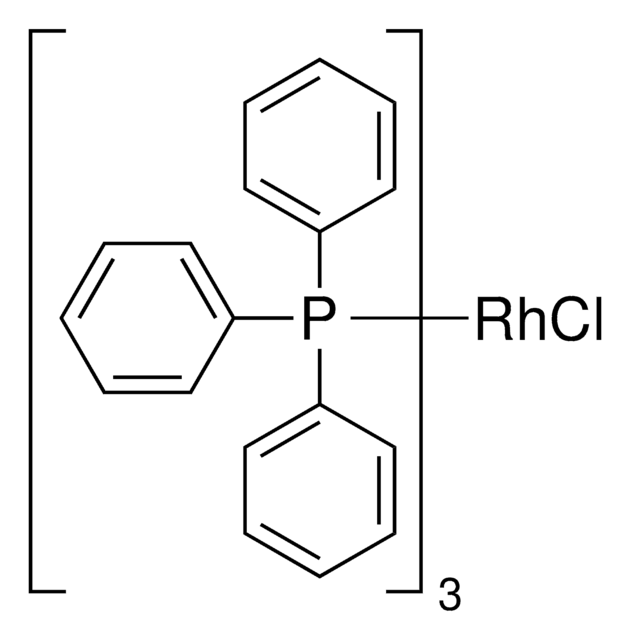
(CAAC-Cy)Rh(COD)Cl is a selective hydrogenation catalyst used for the hydrogenation of a variety of arenes including fluoroarenes giving access to all-cis-multifluorinated cycloalkanes.1
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Mario P Wiesenfeldt et al.
Angewandte Chemie (International ed. in English), 57(27), 8297-8300 (2018-05-24)
We report a method to convert readily available silylated arenes into silylated saturated carbo- and heterocycles by arene hydrogenation. The scope includes alkoxy- and halosilyl substituents. Silyl groups can be derivatized into a plethora of functionalities and find application in
Yu Wei et al.
Journal of the American Chemical Society, 137(29), 9250-9253 (2015-07-15)
Air-stable Rh complexes ligated by strongly σ-donating cyclic (amino)(alkyl)carbenes (CAACs) show unique catalytic activity for the selective hydrogenation of aromatic ketones and phenols by reducing the aryl groups. The use of CAAC ligands is essential for achieving high selectivity and
Mario P Wiesenfeldt et al.
Science (New York, N.Y.), 357(6354), 908-912 (2017-08-12)
All-cis-multifluorinated cycloalkanes exhibit intriguing electronic properties. In particular, they display extremely high dipole moments perpendicular to the aliphatic ring, making them highly desired motifs in material science. Very few such motifs have been prepared, as their syntheses require multistep sequences
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務