推薦產品
形狀
powder
品質等級
儲存溫度
2-8°C
SMILES 字串
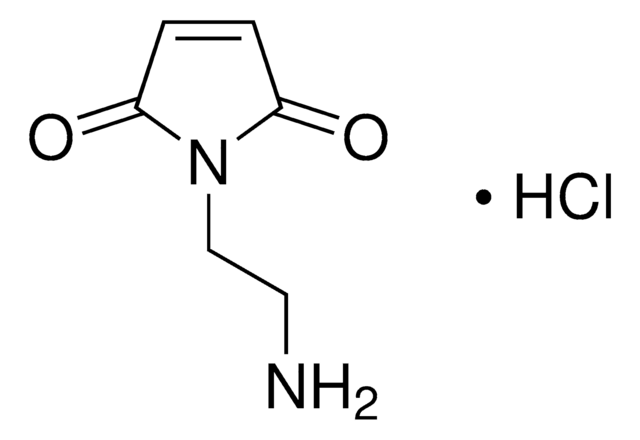
N#CC#CC1=CC=C(N2C(C=CC2=O)=O)C=C1
InChI
1S/C13H6N2O2/c14-9-1-2-10-3-5-11(6-4-10)15-12(16)7-8-13(15)17/h3-8H
InChI 密鑰
CHKKXKRQICWZFF-UHFFFAOYSA-N
應用
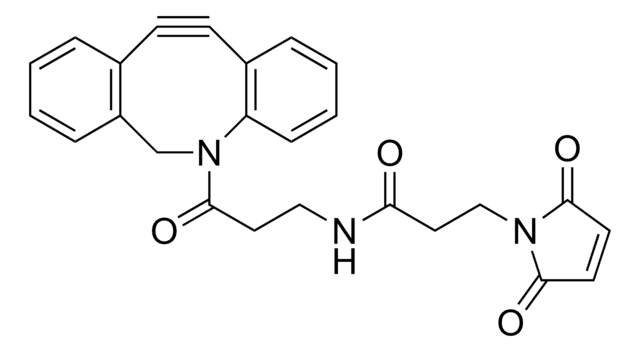
APN-Maleimide is a bifunctional crosslinker for thiol-to-thiol coupling. The coupling can be performed with high selectivity in biological medium using mild reaction conditions. Due to kinetic resolution the first thiol reacts exclusively with the maleimide residue producing the protein-APN conjugate. This conjugate can be readily coupled with thiol-containing molecules.
準備報告
Standard protein labeling procedure (cysteine labeling).
- Dissolve the protein in the appropriate buffer* with pH 6.5-9.0 (e.g. PBS) at 1-10 mg/mL concentration.
- Apply the appropriate amount of the stock solution of the reagent (1-5 molar eq. per free cysteine residue).
- Incubate at room temperature for 10 minutes.
- If necessary, purify the protein-APN conjugate using size exclusion chromatography or ultrafiltration.
- The conjugate can be readily coupled with thiol-containing substrates by incubating the components in aqueous buffer (pH 6.5-9.0) at ambient temperature for 2 hours.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Oleksandr Koniev et al.
Bioconjugate chemistry, 26(9), 1863-1867 (2015-09-04)
Thiols are among the most frequently used functional groups in the field of bioconjugation. While there exists a variety of heterobifunctional reagents that allow for coupling thiols to other functions (e.g., amines, carboxylic acids), there is no specific reagent for
Sergii Kolodych et al.
Bioconjugate chemistry, 26(2), 197-200 (2015-01-24)
Amine-to-thiol coupling is the most common route for the preparation of antibody-drug conjugates (ADC). It is usually achieved by using heterobifunctional reagents possessing an activated ester at one end and a maleimide group at the other. However, maleimide-based conjugates were
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務


![(1R,8S,9s)-双环[6.1.0]壬-4-炔-9-基甲基 N-琥珀酰亚胺碳酸酯 for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)