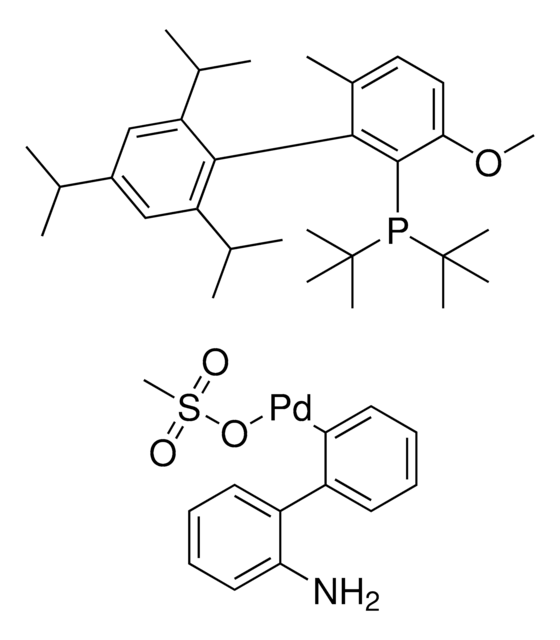
推薦產品
品質等級
化驗
95%
形狀
solid
特點
generation 3
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
環保替代產品評分
old score: 16
new score: 2
Find out more about DOZN™ Scoring
環保替代產品特色
Waste Prevention
Atom Economy
Safer Solvents and Auxiliaries
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
雜質
≤3% acetone
mp
196-241 °C (decomposition)
官能基
phosphine
環保替代類別
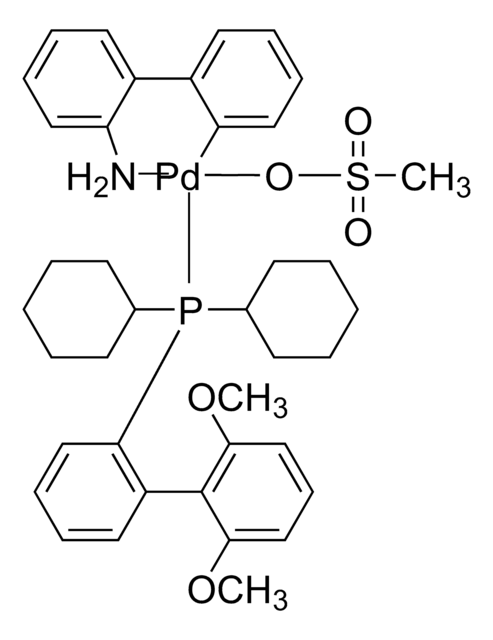
SMILES 字串
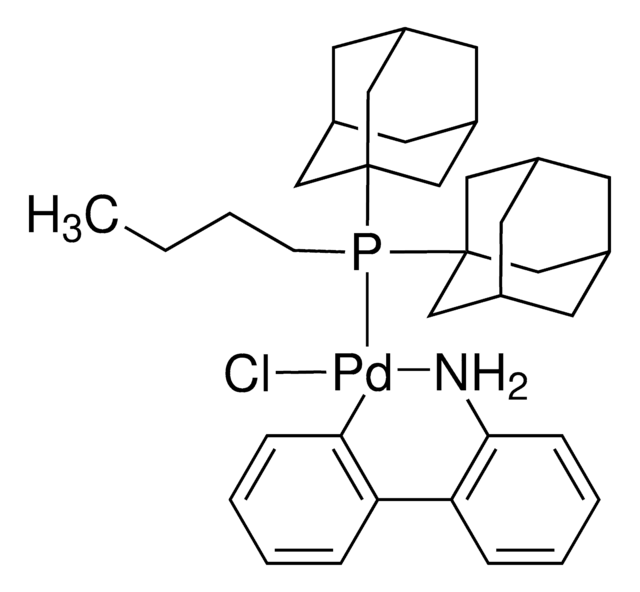
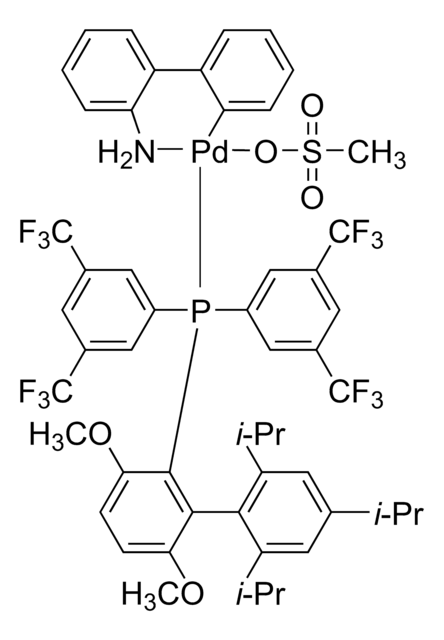
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CCCCP([C@@]34C[C@@H]5C[C@@H](C[C@@H](C5)C3)C4)[C@@]67C[C@@H]8C[C@@H](C[C@@H](C8)C6)C7
InChI
1S/C24H39P.C12H10N.CH4O3S.Pd/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h17-22H,2-16H2,1H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1/t17-,18+,19-,20-,21+,22-,23-,24-;;;
InChI 密鑰
REYVZCOGMIXVNX-DVBMAMJVSA-M
一般說明
應用
- 吡啶羧酸的直接邻位芳基化。
- 催化Suzuki–Miyaura交叉偶联反应,以合成1-杂芳基-3-氮杂双环[3.1.0]己烷。
- 炔烃的钯催化羰基碳全氟烷基化。
- 各种 gem二取代环丙烷的合成过程中成对二(溴基)环丙烷的Suzuki–Miyaura 偶联反应。
- 末端炔烃的溴全氟烷基化。
- 芳香卤化物和炔烃的无铜Sonogashira 偶联反应,以形成 C-C键。
- 有机三氟硼酸盐和芳基卤化物的Suzuki 交叉偶联反应。
法律資訊
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
文章
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Multiple tools have been created to ensure your success with kit set up. Start with the more detailed guide to ensure you are comfortable with all of the steps before using the quick guides on the excel worksheet. Remember that while the technique is new, it is still organic chemistry and so the steps will seem easy once you try just one kit. It is just a new way of approaching something you are already very good at.
KitAlysis™ Suzuki-Miyaura Cross-Coupling Reaction Screening Kit
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務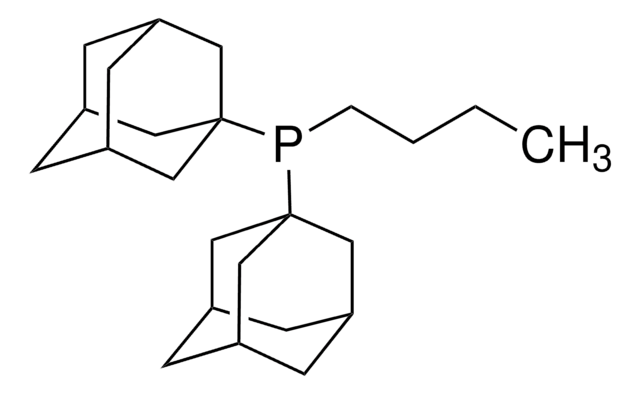

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

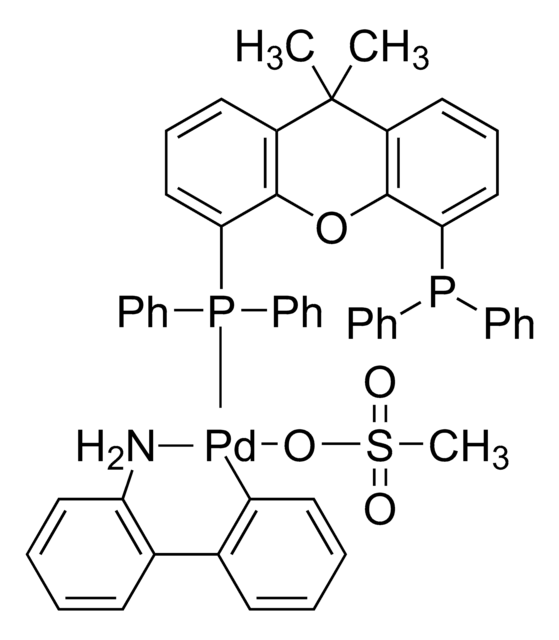
![[(1,3,5,7-四甲基-6-苯基-2,4,6-三氧杂-6-磷杂金刚烷)-2-(2′-氨基-1,1′-联苯)]甲磺酸钯(II)](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)
![氯[(三-TERT-三丁基膦)-2-(2-氨基联苯)]钯(II)](/deepweb/assets/sigmaaldrich/product/structures/100/957/42c5dad6-6197-4fa6-8481-3fe55f0291e9/640/42c5dad6-6197-4fa6-8481-3fe55f0291e9.png)