全部照片(2)
About This Item
經驗公式(希爾表示法):
C10H16NO
CAS號碼:
分子量::
166.24
MDL號碼:
分類程式碼代碼:
12352000
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
形狀
solid
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
87-93 °C
環保替代類別
儲存溫度
2-8°C
SMILES 字串
CC12CC3CC(CC(C3)N1[O])C2
InChI
1S/C10H16NO/c1-10-5-7-2-8(6-10)4-9(3-7)11(10)12/h7-9H,2-6H2,1H3
InChI 密鑰
IBNXYCCLPCGKDM-UHFFFAOYSA-N
相關類別
一般說明
1-Methyl-2-azaadamantane-N-oxyl (1-Me-AZADO), a sterically less hindered nitroxyl radical, is widely used as catalyst. It is chemically stable and exhibits superior catalytic performance.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Find details here.
應用
1-Methyl-2-azaadamantane-N-oxyl (1-Me-AZADO) may be employed as catalyst for the aerobic oxidation of alcohols and sterically hindered alcohols.
Catalytic oxidant for greener oxidation of alcohols under aerobic, solvent-free conditions. Recoverable and recyclable.
Solvent free aerobic oxidation of alcohols with 1-methyl-2-azaadamantane N-oxyl as a recyclable catalyst through phase separation
Solvent free aerobic oxidation of alcohols with 1-methyl-2-azaadamantane N-oxyl as a recyclable catalyst through phase separation
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Solvent free aerobic oxidation of alcohols with 1-methyl-2-azaadamantane N-oxyl as a recyclable catalyst through phase separation.
Kuang Y, et al.
Green Chemistry, 13(7), 1659-1663 (2011)
Efficient oxidation of alcohols electrochemically mediated by azabicyclo-N-oxyls.
Demizu Y, et al.
Tetrahedron Letters, 49(1), 48-52 (2008)
文章
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務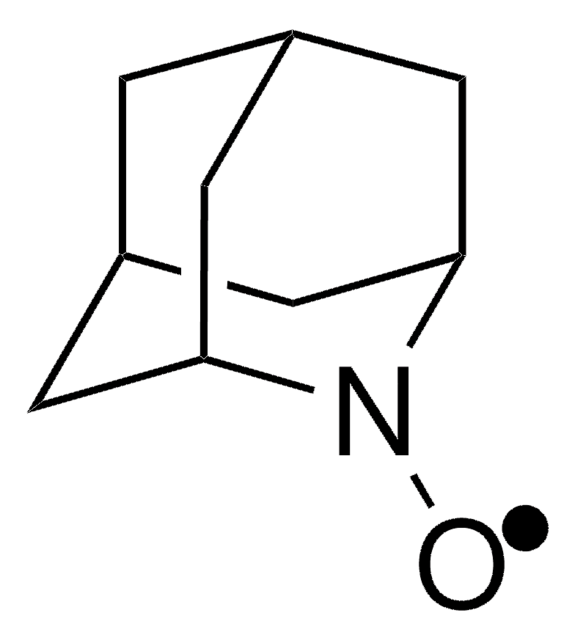

![9-氮杂双环[3.3.1]壬烷N-氧基 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)