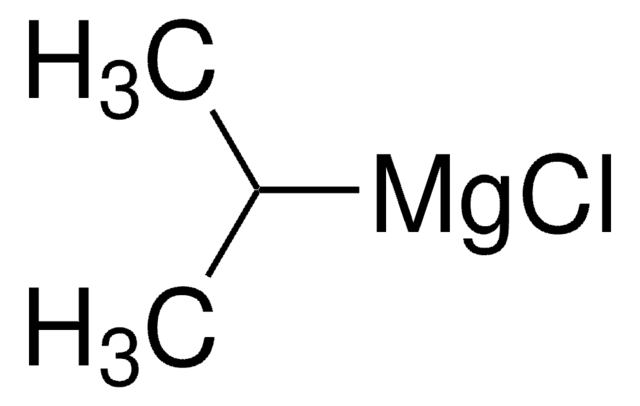
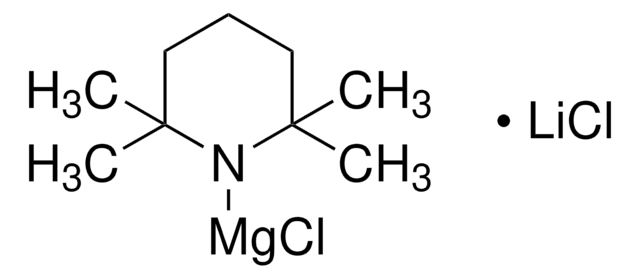
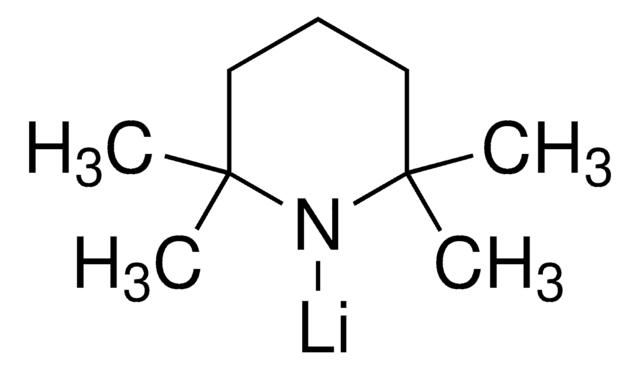
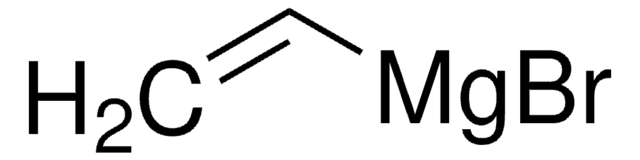
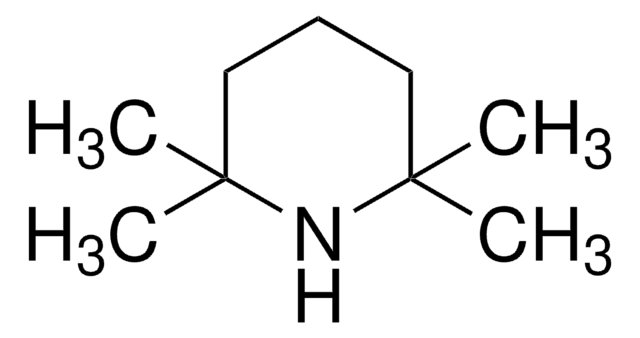
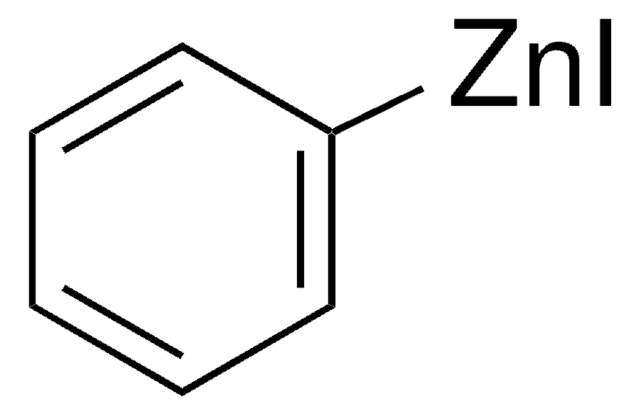
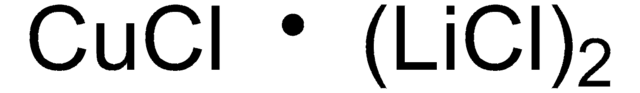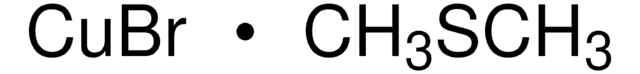推薦產品
形狀
liquid
品質等級
反應適用性
core: copper
reagent type: catalyst
濃度
in anhydrous tetrahydrofuran
密度
0.999 g/mL at 25 °C
SMILES 字串
[Li]Cl.[Li]Cl.[Cu]C#N
InChI
1S/CN.2ClH.Cu.2Li/c1-2;;;;;/h;2*1H;;;/q;;;;2*+1/p-2
InChI 密鑰
QGXKBLXNBYNHBV-UHFFFAOYSA-L
應用
氰化亚铜(I)二(氯化锂)配合物可用于,通过有机锌 和格氏(Grignard)试剂的转移金属化合成有机铜(I)试剂。 也是制备有机铜(I)试剂的有用前体。
訊號詞
Danger
危險分類
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Central nervous system, Respiratory system
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
1.4 °F - closed cup - Solvent
閃點(°C)
-17 °C - closed cup - Solvent
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Regioselective functionalization of trisubstituted pyridines using a bromine-magnesium exchange.
Ren H and Knochel P
Chemical Communications (Cambridge, England), 11(7), 726-728 (2006)
Krause, N.; Gerold, A.
Angewandte Chemie (International Edition in English), 36, 186-186 (1997)
Tomas Hudlicky et al.
The Journal of organic chemistry, 67(25), 8726-8743 (2002-12-07)
Biocatalytic approaches have yielded efficient total syntheses of the major Amaryllidaceae alkaloids, all based on the key enzymatic dioxygenation of suitable aromatic precursors. This paper discusses the logic of general synthetic design for lycoricidine, narciclasine, pancratistatin, and 7-deoxypancratistatin. Experimental details
TMPZnCl?LiCl: A new active selective base for the directed zincation of sensitive aromatics and heteroaromatics.
Mosrin M and Knochel P
Organic Letters, 11(8), 1837-1840 (2009)
Joel M Harris et al.
The Journal of organic chemistry, 68(11), 4371-4381 (2003-05-24)
2,5,6-Trisubstituted piperidines are readily prepared by a combination of an aza-Achmatowicz oxidation of a furyl-substituted benzenesulfonamide followed by a conjugate addition to the resulting 2H-pyridone and subsequent addition of various nucleophiles to a transient N-sulfonyliminium ion. The stereochemistry of the
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 697257-50ML | 4061832786315 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務