推薦產品
化驗
≥97.0% (GC)
光學活性
[α]/D -145±5°, c = 4.47 in chloroform
mp
39-44 °C
官能基
amine
儲存溫度
2-8°C
SMILES 字串
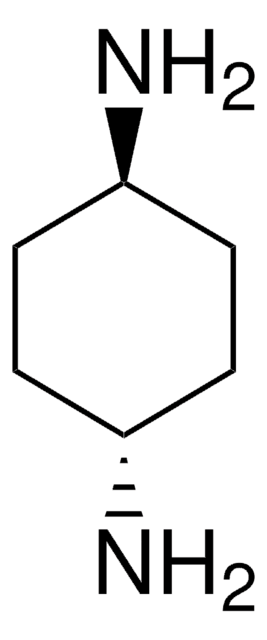
CN[C@@H]1CCCC[C@H]1NC
InChI
1S/C8H18N2/c1-9-7-5-3-4-6-8(7)10-2/h7-10H,3-6H2,1-2H3/t7-,8-/m1/s1
InChI 密鑰
JRHPOFJADXHYBR-HTQZYQBOSA-N
一般說明
(R,R)-(-)-N,N′-Dimethyl-1,2-cyclohexanediamine is a N,N′-dimethylated derivative of enantiopure 1,2-diaminocyclohexane. It can be formed during the hydrolysis of (R3a,R7a)-1,3-(dimethyl)-2-phenyl-2,3,3a,4,5,6,7,7a-octahydro-1H-1,3,2-benzodiazaphosphole 2-oxide using HCl-methanol.
應用
(R,R)-(−)-N,N′-Dimethyl-1,2-cyclohexanediamine can be used:
- As a ligand in the preparation of 4H-benzo[f]imidazo[1,4]diazepin-6-one through multi-component Ullmann coupling reaction.
- In one of the key synthetic steps for the preparation of tricyclic γ-secretase modulators.
- To prepare chiral fluorous diamino-diol proligand, which is used in the synthesis of zirconium metal complexes applicable as catalysts in 1-hexene polymerization.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
165.2 °F - closed cup
閃點(°C)
74 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Microwave-assisted synthesis of 4H-benzo [f] imidazo [1, 4] diazepin-6-ones via a post-Ugi copper-catalyzed intramolecular Ullmann coupling
Li Z, et al.
Tetrahedron Letters, 55(13), 2070-2074 (2014)
An efficient method for the synthesis of N,N'-dimethyl-1, 2-diamines.
Tye H, et al.
Tetrahedron Letters, 43(1), 155-158 (2002)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務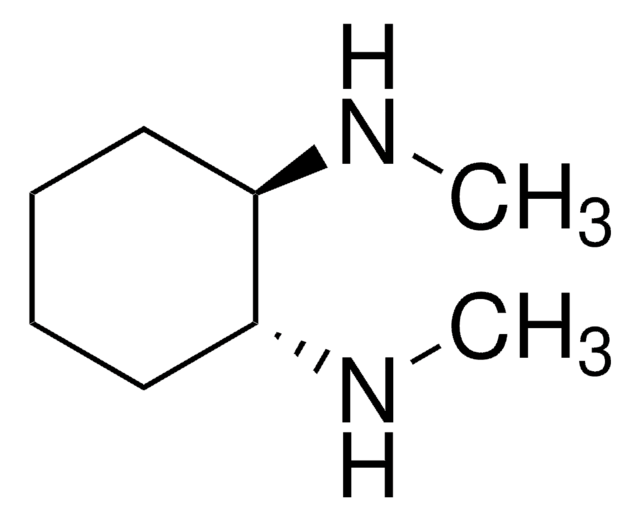






![1,3-双[3,5-双(三氟甲基)苯基]硫脲 95%](/deepweb/assets/sigmaaldrich/product/structures/191/427/0218c99c-65b9-4963-938c-c47a5790dfc5/640/0218c99c-65b9-4963-938c-c47a5790dfc5.png)