推薦產品
品質等級
化驗
95%
形狀
solid
mp
59-64 °C (lit.)
SMILES 字串
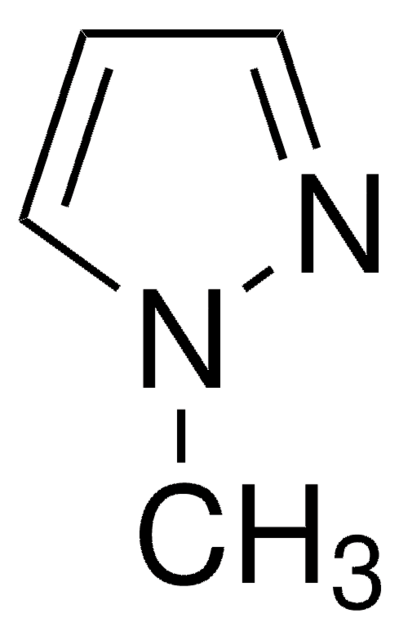
Cn1cc(cn1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C10H17BN2O2/c1-9(2)10(3,4)15-11(14-9)8-6-12-13(5)7-8/h6-7H,1-5H3
InChI 密鑰
UCNGGGYMLHAMJG-UHFFFAOYSA-N
應用
稳定的硼酸替代品,用于Suzuki-Miyaura钯催化交叉偶联
试剂用于
用于制备
- Suzuki-Miyaura交叉偶联反应
- 酯交换反应
用于制备
- 氨基噻唑类(γ)-分泌酶调节剂
- 氨基吡啶-吲哚甲酰胺,作为骨髓增生性疾病治疗的潜在 JAK2 抑制剂
- 吡啶衍生物作为 TGF-β1 和激活素 A 信号传导抑制剂
- MK-2461 类似物作为 c-Met 激酶抑制剂治疗癌症
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
客戶也查看了
Michael Lainchbury et al.
Journal of medicinal chemistry, 55(22), 10229-10240 (2012-10-23)
Inhibitors of checkpoint kinase 1 (CHK1) are of current interest as potential antitumor agents, but the most advanced inhibitor series reported to date are not orally bioavailable. A novel series of potent and orally bioavailable 3-alkoxyamino-5-(pyridin-2-ylamino)pyrazine-2-carbonitrile CHK1 inhibitors was generated
Rudy Ciayadi et al.
Bioorganic & medicinal chemistry letters, 21(18), 5642-5645 (2011-07-26)
Novel inhibitors of TGF-β1 and activin A signalling based on a 2-aryl-4-(3-(pyridin-2-yl)-1H-pyrazol-4-yl)pyridine pharmacophore have been synthesised. Compounds containing phenyl or aromatic nitrogen heterocycle substituents inhibited both types of signalling with HEK-293T cells in culture, with a selectivity preference for TGF-β1.
Direct conversion of pinacol arylboronic esters to aryl triolborates
Li, G.-Q.; et al.
Chemistry Letters (Jpn), 40, 702-704 (2011)
Brijesh Bhayana et al.
Organic letters, 11(17), 3954-3957 (2009-08-12)
A catalyst system for the Suzuki-Miyaura cross-coupling reactions of aryl and vinyl tosylates and mesylates has been developed. This catalyst displays excellent functional group tolerance and allows the coupling of heteroarylboronic acids with aryl tosylates and mesylates to be performed
Thomas Lübbers et al.
Bioorganic & medicinal chemistry letters, 21(21), 6554-6558 (2011-09-20)
We herein report the discovery of a new γ-secretase modulator class with an aminothiazole core starting from a HTS hit (3). Synthesis and SAR of this series are discussed. These novel compounds demonstrate moderate to good in vitro potency in
文章
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![1-[1-(2-Methylphenyl)-1H-pyrazol-4-yl]methanamine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/193/071/8c363ad6-8306-4c4d-b322-749ff2feff6f/640/8c363ad6-8306-4c4d-b322-749ff2feff6f.png)
![4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrrolo[2,3-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/272/176/ea333f93-763d-458c-a328-3969b7d46e5d/640/ea333f93-763d-458c-a328-3969b7d46e5d.png)