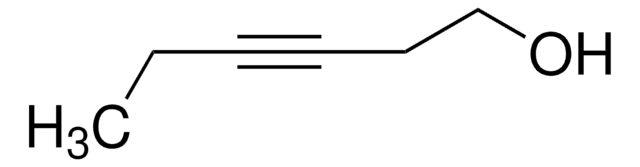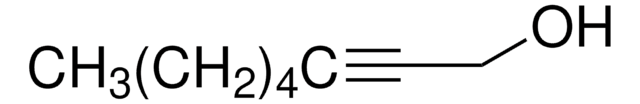全部照片(1)
About This Item
線性公式:
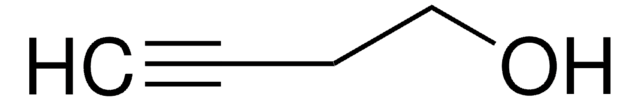
CH3(CH2)3C≡CCH2CH2OH
CAS號碼:
分子量::
126.20
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
折射率
n20/D 1.4569 (lit.)
密度
0.880 g/mL at 25 °C (lit.)
SMILES 字串
CCCCC#CCCO
InChI
1S/C8H14O/c1-2-3-4-5-6-7-8-9/h9H,2-4,7-8H2,1H3
InChI 密鑰
LRZGRGVRZSDRTK-UHFFFAOYSA-N
一般說明
3-Octyn-1-ol is a homopropargylic alcohol that can be prepared from 3-butyn-1-ol and 1-bromobutane.
應用
3-Octyn-1-ol may be used in the preparation of:
- (3Z)-octen-1-ol
- 7-octyn-1-ol
- 3-cis-octenoic acid
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Selective cleavage of dimethylhydrazones to the carbonyl compounds using silica gel and its application in the synthesis of (Z)-9-tetradecenyl acetate.
Mitra RB and Reddy GB.
Synthesis, 1989(09), 694-698 (1989)
Peter Witzgall et al.
Journal of chemical ecology, 31(12), 2923-2932 (2005-12-21)
Analysis of extracts of sex pheromone glands of grapevine moth females Lobesia botrana showed three previously unidentified compounds, (E)-7-dodecenyl acetate and the (E,E)- and (Z,E)-isomers of 7,9,11-dodecatrienyl acetate. This is the first account of a triply unsaturated pheromone component in
Pd/CaCO3 in liquid poly (ethylene glycol)(PEG): an easy and efficient recycle system for partial reduction of alkynes to cis-olefins under a hydrogen atmosphere.
Chandrasekhar S, et al.
Tetrahedron Letters, 45(11), 2421-2423 (2004)
Dongyan Zhang et al.
The Journal of biological chemistry, 277(11), 9127-9132 (2002-01-10)
The degradation of unsaturated fatty acids by beta-oxidation involves Delta(3),Delta(2)-enoyl-CoA isomerases (enoyl-CoA isomerases) that catalyze 3-cis --> 2-trans and 3-trans --> 2-trans isomerizations of enoyl-CoAs and the 2,5 --> 3,5 isomerization of dienoyl-CoAs. An analysis of rat liver enoyl-CoA isomerases
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務