推薦產品
化驗
97%
mp
65-69 °C (lit.)
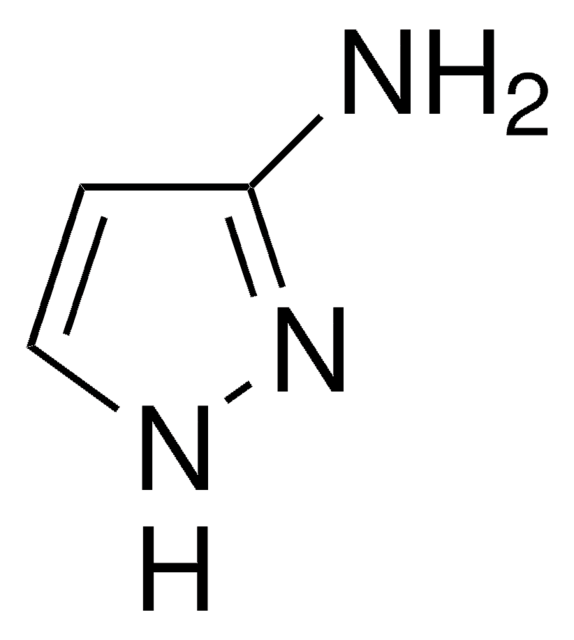
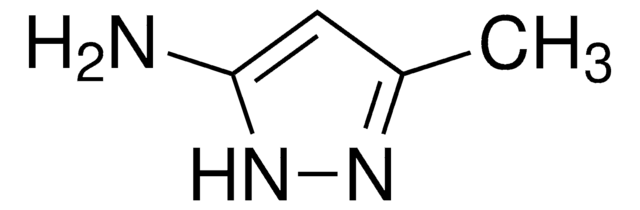
SMILES 字串
Cc1cc(N)n(C)n1
InChI
1S/C5H9N3/c1-4-3-5(6)8(2)7-4/h3H,6H2,1-2H3
InChI 密鑰
ZFDGMMZLXSFNFU-UHFFFAOYSA-N
一般說明
5-Amino-1,3-dimethylpyrazole undergoes cyclocondensation with ethyl acetoacetate to form the corresponding tetrahydropyrazolopyridine derivatives.
應用
5-Amino-1,3-dimethylpyrazole may be used in the preparation of:
- 5-benzamido-1,3-dimethylpyrazole
- diethyl 2-{[(1,3-dimethyl-1H-pyrazol-5-yl)amino]methylene}malonate
- 5-amino-1,3-dimethyl-4-phthalidylpyrazole
- (E)-N-(3,7-dimethylocta-2,6-dienyl)-1,3-dimethyl-1H-pyrazol-5-amine analog(LQFM002)
- 4-isopropyl-1,3-dimethyl-1H-pyrazolo[3,4-b]pyridin-6-ol
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
190.4 °F - closed cup
閃點(°C)
88 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Hiroshi Ochiai et al.
Chemical & pharmaceutical bulletin, 52(9), 1098-1104 (2004-09-02)
A series of 4-anilinopyrazolopyridine derivatives were synthesized and biologically evaluated as inhibitors of phosphodiesterase (PDE4). Chemical modification of 3, a structurally new chemical lead that was found in our in-house library, was focused on 1- and 3-substituents. Full details of
The cyclocondensation of 5?amino?1,3?dimethylpyrazole with ethyl acetoacetate. Synthesis of isomeric pyrazolopyridones.
Ratajczyk JD and Swett LR.
Journal of Heterocyclic Chemistry, 12(3), 517-522 (1975)
1,3-Oxazines and related compounds. II.1) Ring contraction reaction of 1, 3-oxazin-4-one derivatives into 1,2,4-triazoles and pyrazoles.
Yamamoto, et al.
Chemical & Pharmaceutical Bulletin, 26(6), 1825-1831 (1978)
The reaction of o?phthalaldchydic acid with 5?amino?1,3?dimethylpyrazole.
Swett LR and Aynilian GH.
Journal of Heterocyclic Chemistry, 12(6), 1135-1136 (1975)
E A Costa et al.
Life sciences, 92(3), 237-244 (2013-01-09)
The current study describes the synthesis and pharmacological evaluation of (E)-N-(3,7-dimethylocta-2,6-dienyl)-1,3-dimethyl-1H-pyrazol-5-amine (LQFM002), a compound originally designed through a molecular simplification strategy from 4-nerolidylcatechol. LQFM002 was evaluated for preservation of the PLA(2) enzyme inhibitory effects of the lead compound, 4-nerolidylcatechol, using
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務