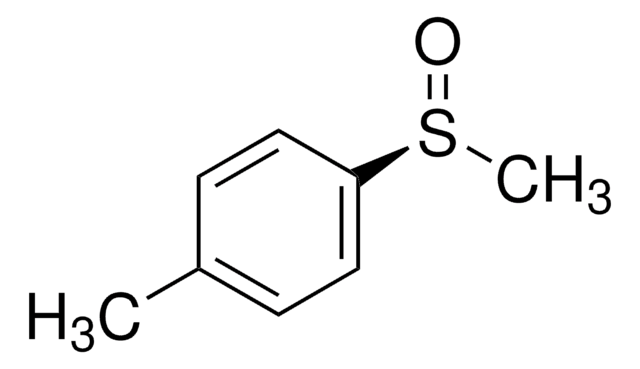
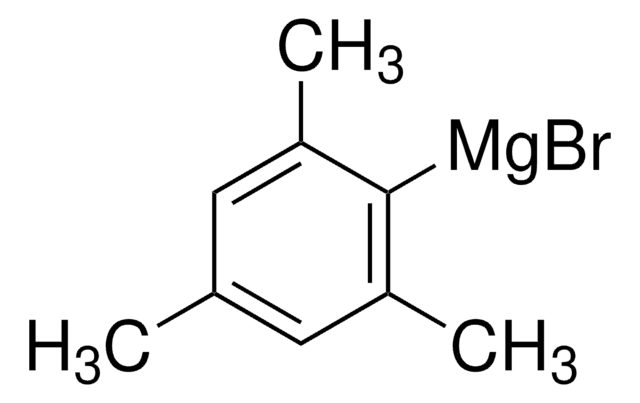
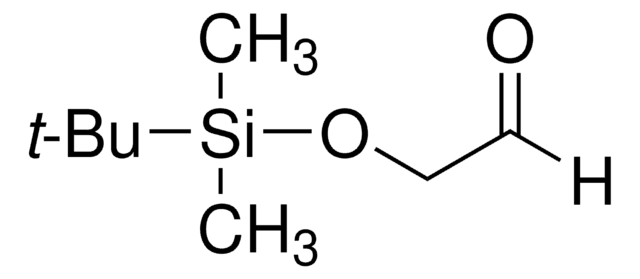
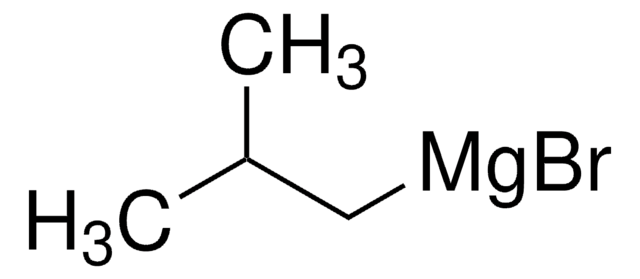
513210
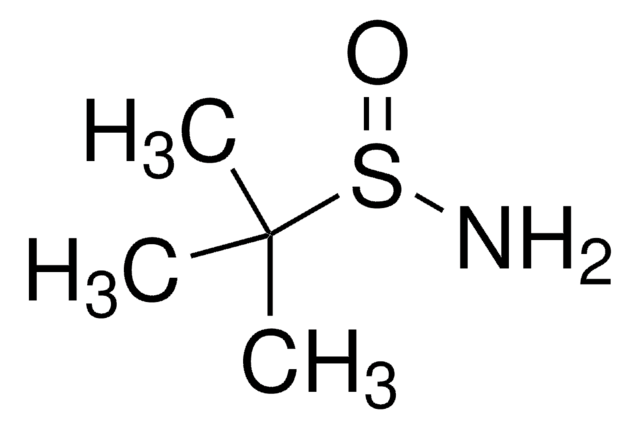
(S)-(−)-2-甲基-2-丙烷亚磺酰胺
97%
同義詞:
(S)-(-)-tert-Butanesulfinamide, (S)-(-)-tert-Butyl sulfinamide, (S)-2-Methyl-2-propanesulfinamide, (S)-tert-Butanesulfinamide, (S)-tert-Butylsulfinamide
About This Item
推薦產品
化驗
97%
光學活性
[α]20/D −4.5°, c = 1 in chloroform
mp
97-101 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)S(N)=O
InChI
1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3/t7-/m0/s1
InChI 密鑰
CESUXLKAADQNTB-ZETCQYMHSA-N
應用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
文章
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務