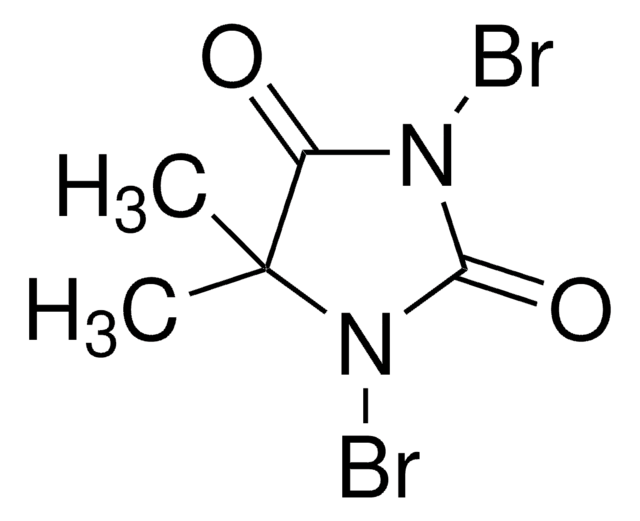
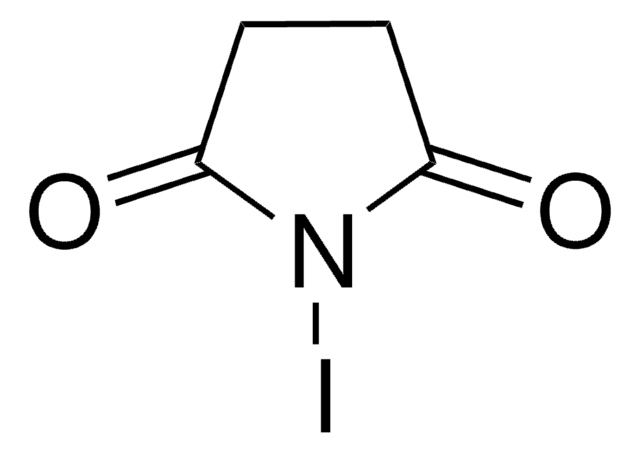
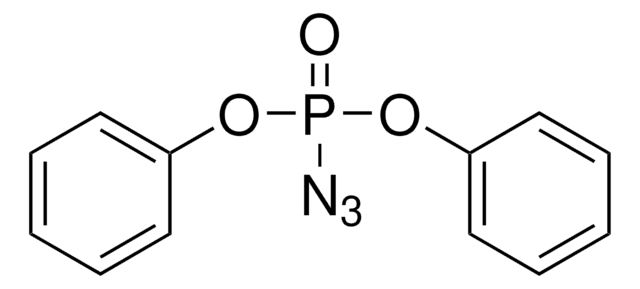
全部照片(1)
About This Item
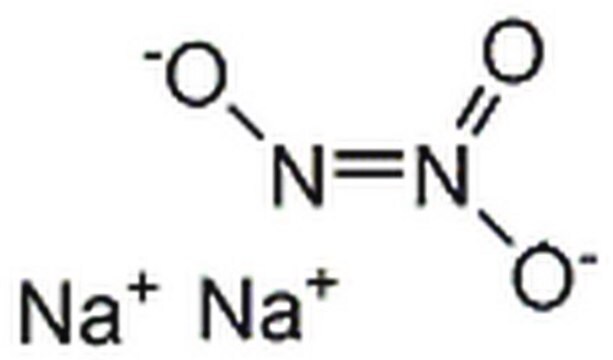
線性公式:
Na2N2O2 · xH2O
CAS號碼:
分子量::
105.99 (anhydrous basis)
MDL號碼:
分類程式碼代碼:
12352302
PubChem物質ID:
NACRES:
NA.22
推薦產品
形狀
solid
品質等級
儲存溫度
2-8°C
SMILES 字串
[Na+].[Na+].[H]O[H].[O-]\N=N\[O-]
InChI
1S/H2N2O2.2Na.H2O/c3-1-2-4;;;/h(H,1,4)(H,2,3);;;1H2/q;2*+1;/p-2
InChI 密鑰
MNTPJDLNJKAOKU-UHFFFAOYSA-L
應用
叔丁基连二次硝酸盐的前体,该盐用于叔丁氧基自由基的低温热生成。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jun Wang et al.
Journal of the American Chemical Society, 131(2), 450-451 (2008-12-23)
A iron-dinitrosyl species ((6)L)Fe(NO)(2) (2), generated from nitrogen monoxide (*NO) binding to its related iron(II)-mononitrosyl complex ((6)L)Fe(NO) (1), efficiently effects reductive coupling of two *NO molecules to release nitrous oxide (N(2)O), when Cu(+) ion and 2 equiv acid are added;
The structure of the hyponitrite species in a heme Fe-Cu binuclear center.
Constantinos Varotsis et al.
Angewandte Chemie (International ed. in English), 46(13), 2210-2214 (2007-02-14)
Gregory A Poskrebyshev et al.
Journal of the American Chemical Society, 126(3), 891-899 (2004-01-22)
All major properties of the aqueous hyponitrite radicals (ONNO- and ONNOH), the adducts of nitric oxide (NO) and nitroxyl (3NO- and 1HNO), are revised. In this work, the radicals are produced by oxidation of various hyponitrite species in the 2-14
Nan Xu et al.
Inorganic chemistry, 49(14), 6253-6266 (2010-07-30)
Nitric oxide (NO) and its derivatives such as nitrite and hyponitrite are biologically important species of relevance to human health. Much of their physiological relevance stems from their interactions with the iron centers in heme proteins. The chemical reactivities displayed
Nan Xu et al.
Journal of the American Chemical Society, 131(7), 2460-2461 (2009-02-05)
The coupling of two nitric oxide (NO) molecules in heme active sites is an important contributor to the conversion of NO to nitrous oxide (N(2)O) by heme-containing enzymes. Several formulations for the presumed heme-Fe{N(2)O(2)}(n-) intermediates have been proposed previously, however
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務