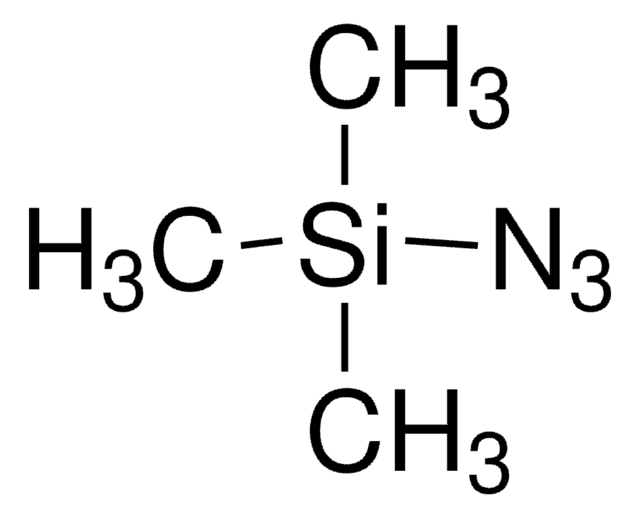About This Item
推薦產品
品質等級
化驗
97%
形狀
liquid
反應適用性
reaction type: click chemistry
折射率
n20/D 1.551 (lit.)
bp
157 °C/0.17 mmHg (lit.)
密度
1.277 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
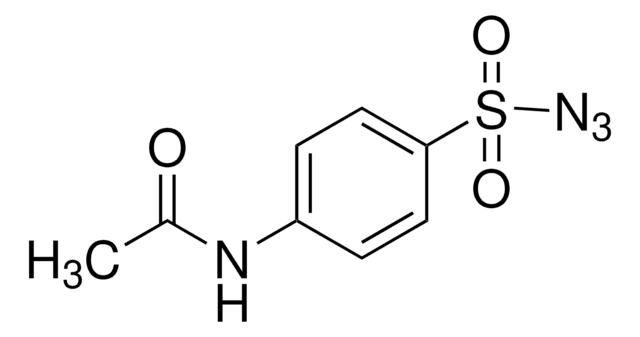
SMILES 字串
[N-]=[N+]=NP(=O)(Oc1ccccc1)Oc2ccccc2
InChI
1S/C12H10N3O3P/c13-14-15-19(16,17-11-7-3-1-4-8-11)18-12-9-5-2-6-10-12/h1-10H
InChI 密鑰
SORGEQQSQGNZFI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
233.6 °F - closed cup
閃點(°C)
112 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
文章
Acid Halogenation Reagents
The generation of an acid chloride is an obvious way to activate the carboxy group for amide bond formation. However, practical application of acid chlorides in peptide synthesis is restricted, because they are prone to side reactions and racemization.
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)


![1,4-二叠氮双环[2.2.2]辛烷 ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)