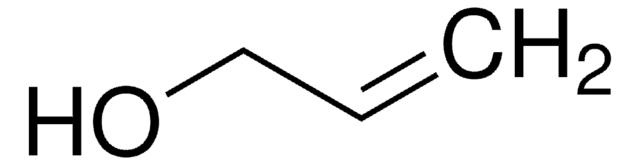
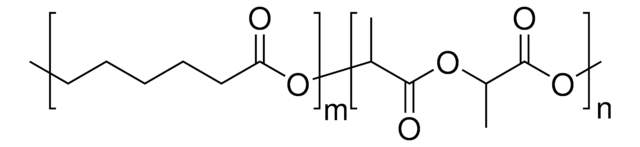
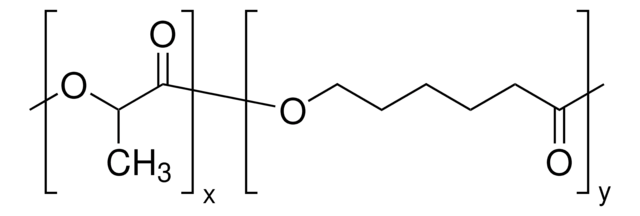
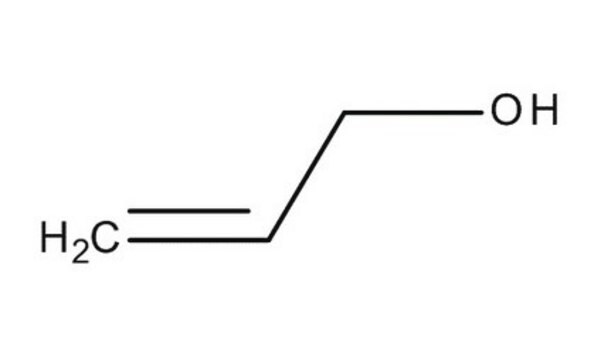
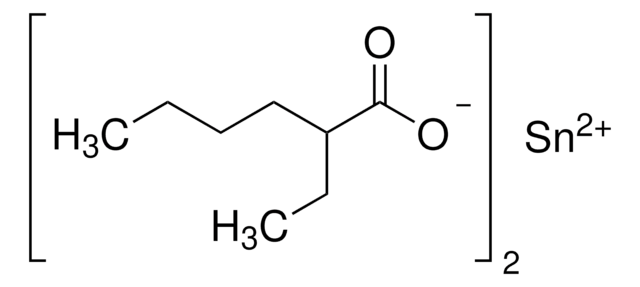
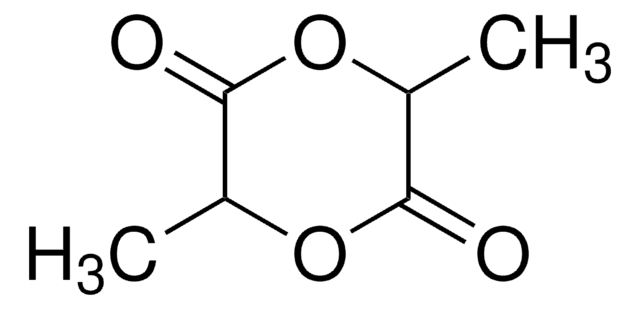
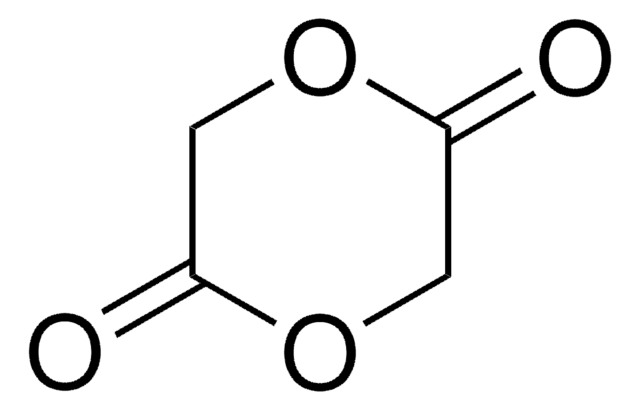
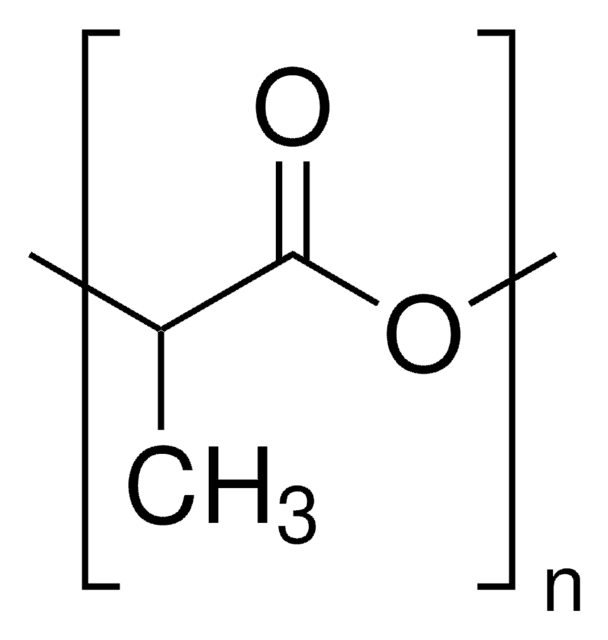
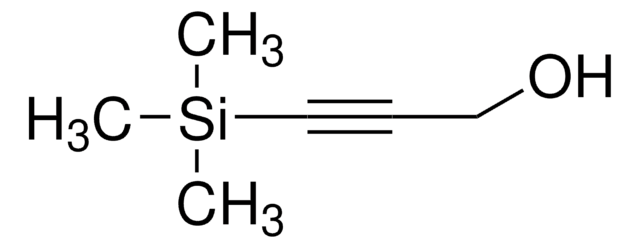
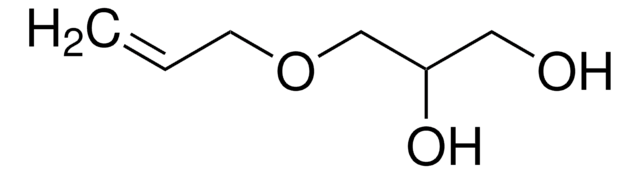
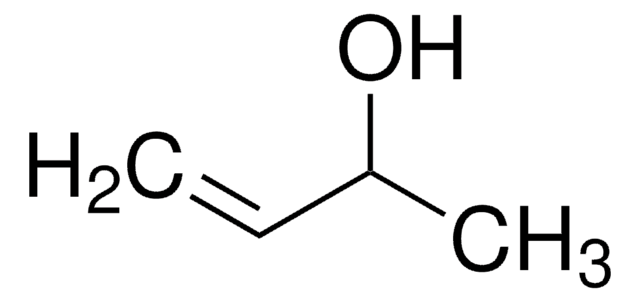
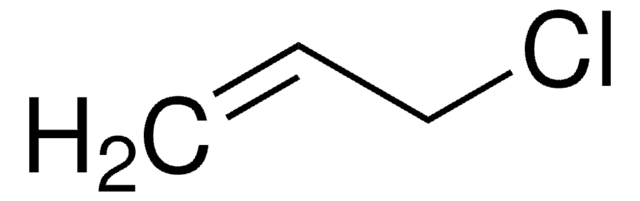
推薦產品
應用
用于诱导肝脏损坏的小鼠模型,该模型用于研究肝细胞毒性和肝干细胞介导修复的机制。
包裝
訊號詞
Danger
危險分類
Acute Tox. 2 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
71.6 °F - closed cup
閃點(°C)
22 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Hydrosilylation of allyl alcohol with [HSiMe2OSiO1.5]8: octa (3-hydroxypropyldimethylsiloxy) octasilsesquioxane and its octamethacrylate derivative as potential precursors to hybrid nanocomposites.
Zhang C and Laine RM.
Journal of the American Chemical Society, 122(29), 6979-6988 (2000)
Gregory M Mullen et al.
Journal of the American Chemical Society, 136(17), 6489-6498 (2014-04-08)
Partial oxidation of alcohols is a topic of great interest in the field of gold catalysis. In this work, we provide evidence that the partial oxidation of allyl alcohol to its corresponding aldehyde, acrolein, over oxygen-precovered gold surfaces occurs via
Deoxydehydration of glycerol to allyl alcohol catalyzed by rhenium derivatives.
Canale V, et al.
Catalysis Science & Technology, 4(10), 3697-3704 (2014)
James Y Hamilton et al.
Journal of the American Chemical Society, 135(3), 994-997 (2012-12-22)
The first example of Ir-catalyzed asymmetric substitution reaction with vinyl trifluoroborates is described. The direct reaction between branched, racemic allylic alcohols and potassium alkenyltrifluoroborates proceeded with high site selectivity and excellent enantioselectivity (up to 99%) mediated by an Ir-(P,olefin) complex.
Johanna M Larsson et al.
Journal of the American Chemical Society, 135(1), 443-455 (2012-12-04)
The mechanism of the palladium-catalyzed synthesis of allylic silanes and boronates from allylic alcohols was investigated. (1)H, (29)Si, (19)F, and (11)B NMR spectroscopy was used to reveal key intermediates and byproducts of the silylation reaction. The tetrafluoroborate counterion of the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務