全部照片(1)
About This Item
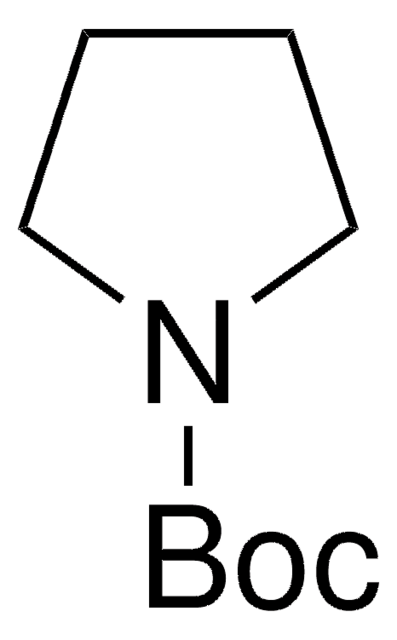
經驗公式(希爾表示法):
C9H13NO2
CAS號碼:
分子量::
167.21
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
98%
形狀
liquid
折射率
n20/D 1.4685 (lit.)
bp
91-92 °C/20 mmHg (lit.)
密度
1 g/mL at 25 °C (lit.)
SMILES 字串
CC(C)(C)OC(=O)n1cccc1
InChI
1S/C9H13NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-7H,1-3H3
InChI 密鑰
IZPYBIJFRFWRPR-UHFFFAOYSA-N
一般說明
N-Boc-吡咯是 N-保护的吡咯。它与对映体纯丙二烯-1,3-二羧酸酯进行 Diels-Alder 反应,形成内部加合物,在两个新生成的立体中心的保留构型。它还与苯基二乙酸甲酯进行环丙烷化,形成单环丙烷和二环丙烷。据报道,其 Ir 催化的 C-H 硼酸化,随后与 3-氯噻吩交叉偶联形成双杂环。
應用
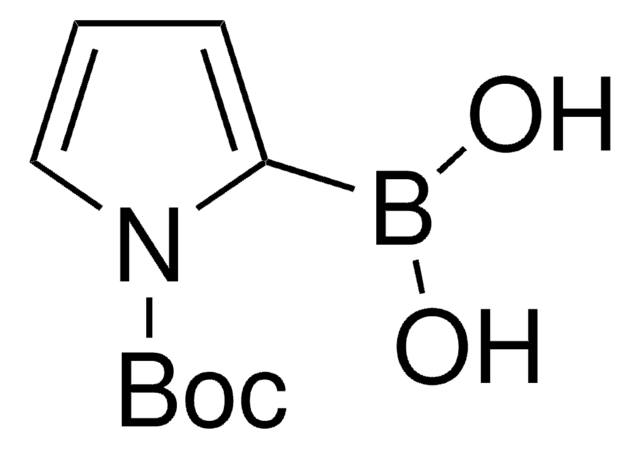
通过用 n-BuLi 处理,随后与硼酸三甲酯反应, N-Boc-吡咯可用于合成 1-(叔丁氧基羰基)-1H-吡咯-2-基硼酸。
它可以用作以下合成中的原料:
它可以用作以下合成中的原料:
- 托品衍生物
- N-BOC-2-(4-甲氧基苯基)吡咯
- N-boc-吡咯-2-基硼酸
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
167.0 °F - closed cup
閃點(°C)
75 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Synthetic approaches to enantiomerically pure 8-azabicyclo [3.2. 1] octane derivatives.
Pollini GP, et al.
Chemical Reviews, 106(6), 2434-2454 (2006)
Huw M L Davies et al.
Chemical Society reviews, 38(11), 3061-3071 (2009-10-23)
The metal catalyzed reactions of diazo compounds have been broadly used in organic synthesis. The resulting metal-carbenoid intermediates are capable of undergoing a range of unconventional reactions, and due to their high energy, they are ideal for initiating cascade sequences
Recent progress in the synthesis of five-membered heterocycle boronic acids and esters.
Primas N, et al.
Tetrahedron, 66(41), 8121-8136 (2010)
Nikola Basarić et al.
Organic & biomolecular chemistry, 3(15), 2755-2761 (2005-07-21)
Two fluorescent off-on Ca2+ indicators based on APTRA (o-aminophenol-N,N,O-triacetic acid) as low-affinity ligand for Ca2+ and BODIPY(4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) as a fluorophore were synthesized. The new BODIPY-APTRA compounds absorb in the visible spectrum, with absorption maxima from 505 nm to 570 nm
CuO/SiO2 as a simple, effective and recoverable catalyst for alkylation of indole derivatives with diazo compounds.
Fraile JM, et al.
Organic & Biomolecular Chemistry, 11(26), 4327-4332 (2013)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務