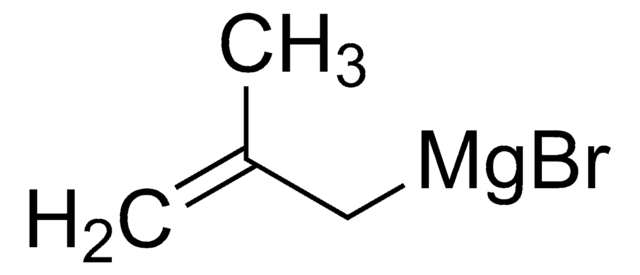
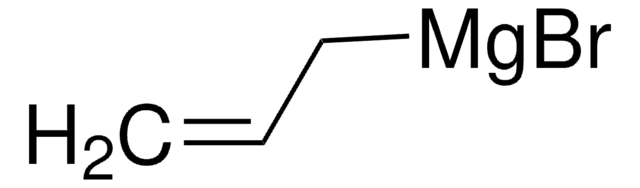
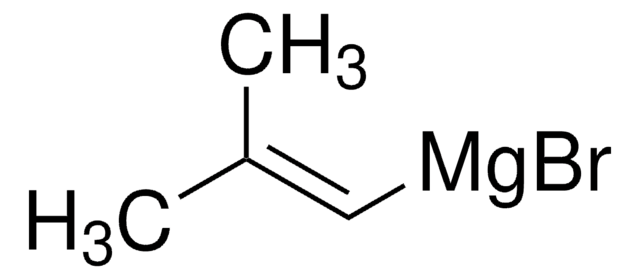
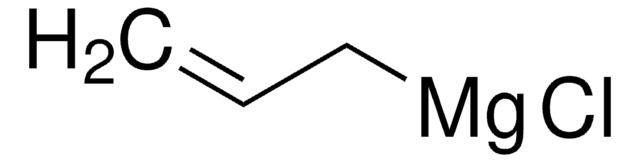
推薦產品
反應適用性
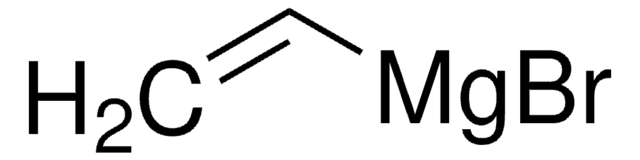
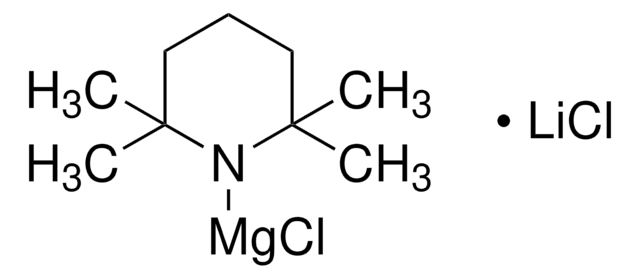
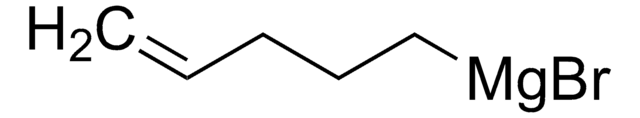
reaction type: Grignard Reaction
濃度
0.5 M in THF
bp
65-67 °C
密度
0.915 g/mL at 25 °C
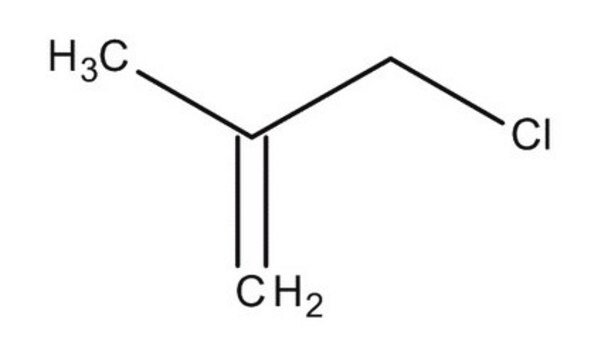
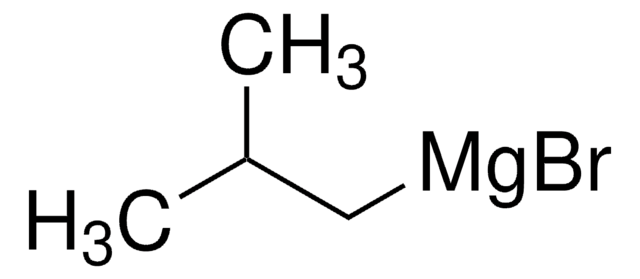
SMILES 字串
CC(=C)C[Mg]Cl
InChI
1S/C4H7.ClH.Mg/c1-4(2)3;;/h1-2H2,3H3;1H;/q;;+1/p-1
InChI 密鑰
BJVFGWYBOLMUEM-UHFFFAOYSA-M
應用
2-Methylallylmagnesium chloride is a general Grignard reagent used in the synthesis of (−)-aplysin, acutumine, dimedol and allyldicyclopentadienyltitanium(III) complexes.
包裝
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
法律資訊
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 2
標靶器官
Respiratory system
安全危害
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 3
閃點(°F)
-5.8 °F - closed cup
閃點(°C)
-21 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Allyldicyclopentadienyltitanium (III) and (DI) methylallyl homologues.
Martin H A and Jellinek F
Journal of Organometallic Chemistry, 8(1), 115-128 (1967)
The total synthesis of (−)-aplysin via a lithiation?borylation?propenylation sequence.
Fletcher C J, et al.
Tetrahedron, 68(37), 7598-7604 (2012)
B List et al.
Proceedings of the National Academy of Sciences of the United States of America, 95(26), 15351-15355 (1998-12-23)
The synthesis of novel fluorogenic retro-aldol substrates for aldolase antibody 38C2 is described. These substrates are efficiently and specifically processed by antibody aldolases but not by natural cellular enzymes. Together, the fluorogenic substrates and antibody aldolases provide reporter gene systems
?Classical carbonyl reactivity enables a short synthesis of the core structure of acutumine?
Moreau.JR and Sorensen.JE
Tetrahedron, 63(28), 6446-6453 (2007)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務