全部照片(1)
About This Item
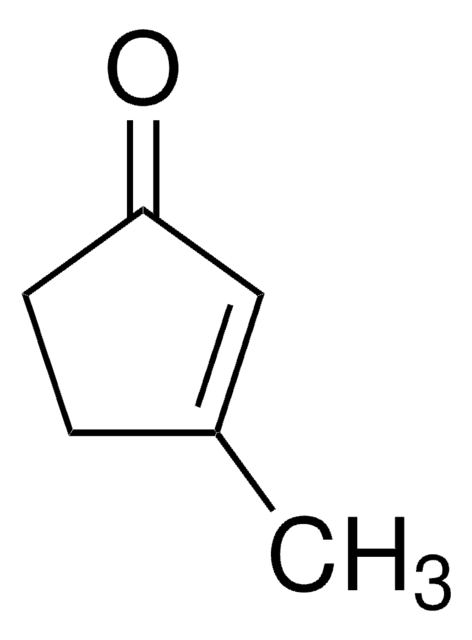
線性公式:
C2H5OC5H5(=O)
CAS號碼:
分子量::
126.15
Beilstein:
2041482
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.492 (lit.)
bp
60-63 °C/0.3 mmHg (lit.)
密度
1.062 g/mL at 25 °C (lit.)
官能基
ether
ketone
SMILES 字串
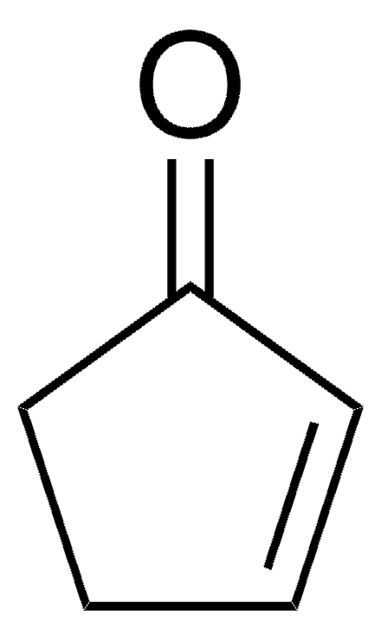
CCOC1=CC(=O)CC1
InChI
1S/C7H10O2/c1-2-9-7-4-3-6(8)5-7/h5H,2-4H2,1H3
InChI 密鑰
SUQNVCCJLBQVEI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
3-Ethoxy-2-cyclopentenone (3-Ethoxy-2-cyclopenten-1-one, 3-Ethoxycyclopentenone) is a cyclic β-alkoxy α,β-unsaturated ketone. Its manganese(III) acetate based tandem oxidation has been investigated. Its various physical properties such as density, refractive index and boiling point have been reported.
應用
3-Ethoxy-2-cyclopentenone (3-Ethoxy-2-cyclopenten-1-one) may be used as a starting material in the synthesis of the following:
- allylic cyclopentenyl alcohols like 2-cyclopentenol, 3-methyl-2-cyclopentenol and 3-methoxymethaoxymethyl-2-cyclopentenol
- 3-aminocyclopent-2-en-1-one
- (E)-5-(((2S*,3S*)-3-((benzyloxy)methyl)oxiran-2- yl)methylene)-3-ethoxycyclopent-2-enone, an γ,δ -epoxy-α,β-unsaturated cyclic enone
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
客戶也查看了
Manganese (III) acetate based tandem oxidation of various α and β-alkoxy α, β-unsaturated ketones.
Tanyeli C, et al.
Tetrahedron, 58(50), 9983-9988 (2002)
An efficient synthesis of 3-aminocyclopent-2-en-1-one.
Kikani BB, et al.
Synthesis, 2, 176-176 (1991)
596144
CorpBase ID (for auto-filling citation data) null
Palladium-catalyzed rearrangements of 2-cyclopentenyloxypyrimidines in the preparation of pyrimidine carbonucleosides.
Falck-Pedersen ML, et al.
Acta Chemica Scandinavica, 47, 72-72 (1993)
Fumihiko Yoshimura et al.
Organic & biomolecular chemistry, 10(28), 5431-5442 (2012-06-19)
We developed a new method for stereoselective construction of an all-carbon quaternary stereogenic center on a carbocyclic ring based on regio- and stereoselective S(N)2' alkylation reactions of γ,δ-epoxy-α,β-unsaturated cyclic ketones. Treatment of the ketones, which were readily prepared in enantiomerically
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務