全部照片(1)
About This Item
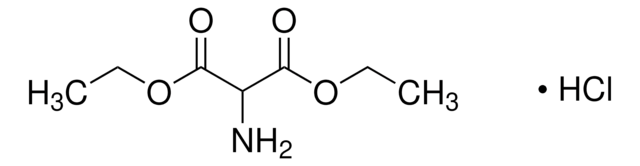
線性公式:
NH2CH(COOCH3)2 · HCl
CAS號碼:
分子量::
183.59
Beilstein:
3696467
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
形狀
crystals
mp
160 °C (dec.) (lit.)
溶解度
water: soluble 25 mg/mL, clear, colorless to faintly yellow
官能基
amine
ester
SMILES 字串
Cl[H].COC(=O)C(N)C(=O)OC
InChI
1S/C5H9NO4.ClH/c1-9-4(7)3(6)5(8)10-2;/h3H,6H2,1-2H3;1H
InChI 密鑰
QWNDKNJSEWOEDM-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Dimethyl aminomalonate hydrochloride is a hydrochloride salt of a dialkyl aminomalonate.
應用
Dimethyl aminomalonate hydrochloride (aminomalonic acid dimethyl ester hydrochloride) may be used in synthesis of methyl 3-phenyl-5-hydantoincarboxylate and Boc-Leu-Ama(OMe)2(Boc= tert-butyloxycarbonyl, Leu= leucine, Ama= aminomalonic acid). It may be used as starting reagent in the synthesis of the following:
- (R,S)-2-phenethylcysteine hydrochloride
- dimethyl 2,2,2-polynitroalkylnitroaminonitromalonate
- spirotryprostatin B
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Synthesis of Nitro Compounds Starting with Dialkyl Aminomalonates.
Ishchenko MA, et al.
Russ. J. Org. Chem., 37(2), 194-197 (2001)
D Krumme et al.
FEBS letters, 436(2), 209-212 (1998-10-22)
Novel peptides containing the sequence -Pro-Leu-Ama(NHOH)- were synthesized and characterized by spectroscopic techniques. Their inhibitory properties towards the activated form of native human gelatinase B (MMP-9) and the catalytic domain of neutrophil collagenase (cdMMP-8) were determined. The most effective inhibitor
Alpha-alkylcysteines as inhibitors for carboxypeptidase A. Synthesis, evaluation, and implication for inhibitor design strategy.
Lee HS and Kim DH.
Bull. Korean Chem. Soc., 23(4), 593-598 (2002)
Heterocyclizations, XIV. 1,3,5,7,-Tetraoxoperhydroimidazo[1,5-c]imidazole, a Novel Bridgehead Nitrogen Ureide
Capuano L, et al.
Chemische Berichte, 107(10), 3237-3245 (1974)
Oxindole as starting material in organic synthesis.
Ziarani GM, et al.
ARKIVOC (Gainesville, FL, United States), 1, 470-535 (2013)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務