565849
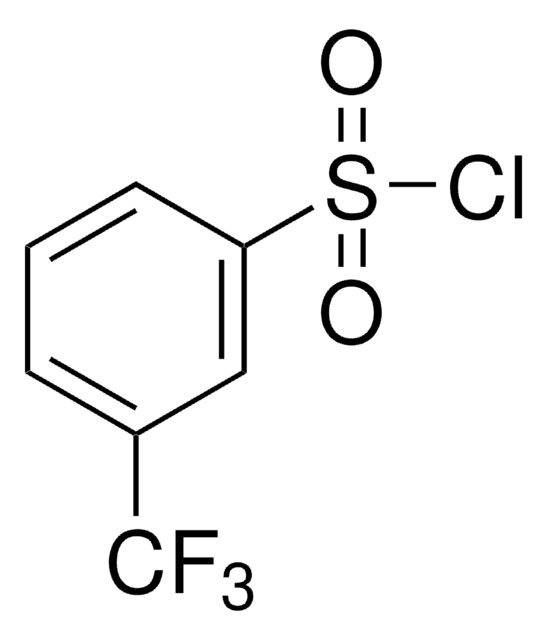
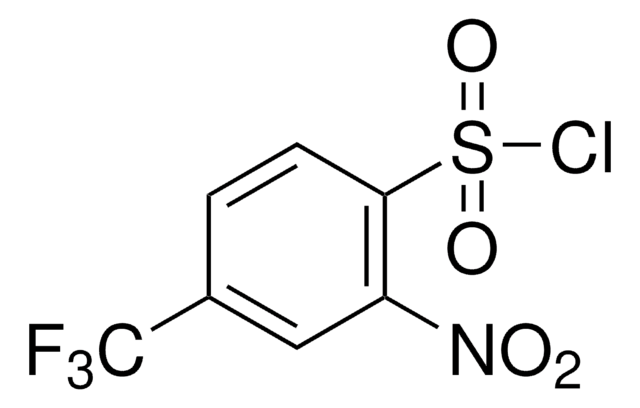
4-(三氟甲基)苯磺酰氯
97%
同義詞:
α,α,α-Trifluoro-p-toluenesulfonyl chloride, 4-(Trifluoromethyl)benzene-1-sulfonyl chloride, [4-(Trifluoromethyl)phenyl]sulfonyl chloride, [p-(Trifluoromethyl)phenyl]sulfonyl chloride, p-Trifluoromethylbenzenesulfonyl chloride
登入查看組織和合約定價
全部照片(2)
About This Item
線性公式:
CF3C6H4SO2Cl
CAS號碼:
分子量::
244.62
Beilstein:
2113604
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
mp
30-34 °C (lit.)
SMILES 字串
FC(F)(F)c1ccc(cc1)S(Cl)(=O)=O
InChI
1S/C7H4ClF3O2S/c8-14(12,13)6-3-1-5(2-4-6)7(9,10)11/h1-4H
InChI 密鑰
OZDCZHDOIBUGAJ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
4-(Trifluoromethyl)benzenesulfonyl chloride may be used to synthesize β-arylated thiophenes and 2,5-diarylated pyrrolevia palladium catalyzed desulfitative arylation of thiophenes and pyrroles, respectively.
4-(Trifluoromethyl)benzenesulfonyl chloride and N-vinylpyrrolidinone in acetonitrile can undergo photo-irradiation with visible light in the presence of Ir(ppy)2(dtbbpy)PF6 ([4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis[2-(2-pyridinyl-N)phenyl-C]iridium(III)hexafluorophosphate) and disodium phosphate to give the corresponding E-vinyl sulfone.
4-(Trifluoromethyl)benzenesulfonyl chloride and N-vinylpyrrolidinone in acetonitrile can undergo photo-irradiation with visible light in the presence of Ir(ppy)2(dtbbpy)PF6 ([4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis[2-(2-pyridinyl-N)phenyl-C]iridium(III)hexafluorophosphate) and disodium phosphate to give the corresponding E-vinyl sulfone.
注意
低熔点固体
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
安全危害
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
>230.0 °F
閃點(°C)
> 110 °C
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
"Benzenesulfonyl chlorides: new reagents for access to alternative regioisomers in palladium-catalysed direct arylations of thiophenes"
Yuan K, et al.
Chemical Science, 5(1), 392-396 (2014)
"C-H Functionalization of Enamides: Synthesis of ?-Amidovinyl Sulfones via Visible-Light Photoredox Catalysis"
Jiang H, et al.
Advanced Synthesis & Catalysis, 355(4), 809-813 (2013)
Palladium-Catalysed Direct Desulfitative Arylation of Pyrroles using Benzenesulfonyl Chlorides as Alternative Coupling Partners.
Jin R, et al.
Advanced Synthesis & Catalysis, 356(18), 3831-3841 (2014)
文章
Aryl sulfonyl chloride derivatives are frequently used in parallel synthesis to synthesize sulfonamides and sulfonate linkages.
Aryl Sulfonyl Chloride Derivatives
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務