推薦產品
品質等級
化驗
97%
mp
97-99 °C (lit.)
溶解度
chloroform: soluble 25 mg/mL, clear, yellow
官能基
bromo
SMILES 字串
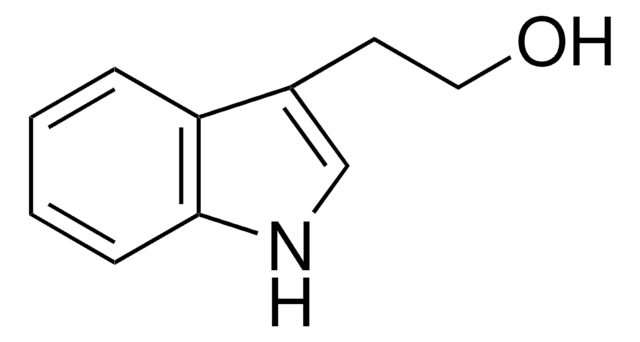
BrCCc1c[nH]c2ccccc12
InChI
1S/C10H10BrN/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2
InChI 密鑰
NTLAICDKHHQUGC-UHFFFAOYSA-N
一般說明
3-(2-Bromoethyl)indole is a halogenated heterocyclic building block.
應用
3-(2-Bromoethyl)indole may be used in the synthesis of:
- β-carboline derivatives
- 6,7-dihydro-12H-indolo[2,3-a] pyridocolinium bromide
- N-(2-(3-indolyl)ethyl)aza-12-crown-4
- N-(2-(3-Indolyl)ethyl)aza-15-crown-5
- N-(2-(3-Indolyl)ethyl)aza-18-crown-6
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Jiaxin Hu et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5121-5126 (2002-04-12)
Feeble forces play a significant role in the organization of proteins. These include hydrogen bonding, hydrophobic interactions, salt bridge formation, and steric interactions. The alkali metal cation-pi interaction is a force of potentially profound importance but its consideration in biology
The synthesis of ?-carboline derivatives-I: A synthesis of some 12 H-indolo [2, 3-a] pyridocolinium salts, including flavopereirine.
Ban Y and Seo M.
Tetrahedron, 16(1), 5-10 (1961)
The synthesis of beta-carboline derivatives. VII. The isolation of the possible intermediate in the condensation of 3-(2-bromoethyl)indole and 2-halogenopyridine.
Y Ban et al.
Chemical & pharmaceutical bulletin, 13(8), 931-934 (1965-08-01)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務