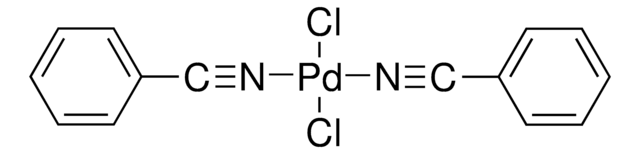
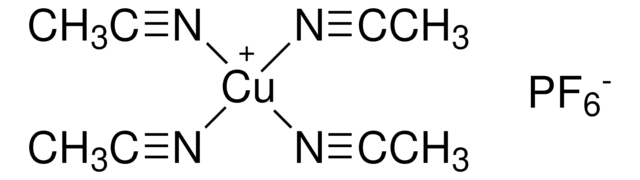
About This Item
推薦產品
形狀
solid
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
core: palladium
reagent type: ligand
mp
230 °C (dec.) (lit.)
SMILES 字串
[Pd++].CC#N.CC#N.CC#N.CC#N.F[B-](F)(F)F.F[B-](F)(F)F
InChI
1S/4C2H3N.2BF4.Pd/c4*1-2-3;2*2-1(3,4)5;/h4*1H3;;;/q;;;;2*-1;+2
InChI 密鑰
YWMRPVUMBTVUEX-UHFFFAOYSA-N
相關類別
一般說明
應用
反应物:
- 由于弱配位乙腈配体而作为金属来源作用的反应
前体:
- 树枝状 SCS-钳形钯配合物的合成
- 短声配体钯配合物
- 用于 Heck 交叉偶联 、Suzuki 交叉偶联和醛烯化反应的双钯催化剂
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Inhalation
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
文章
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務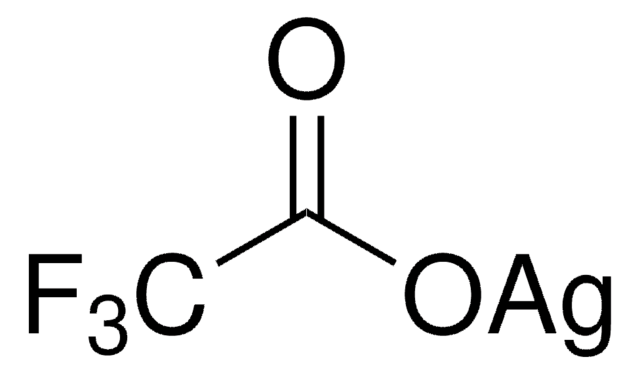
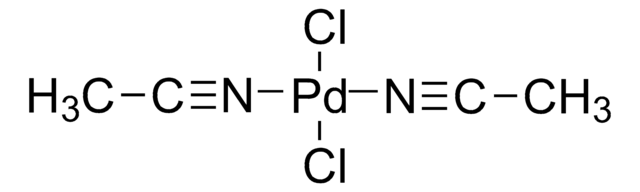

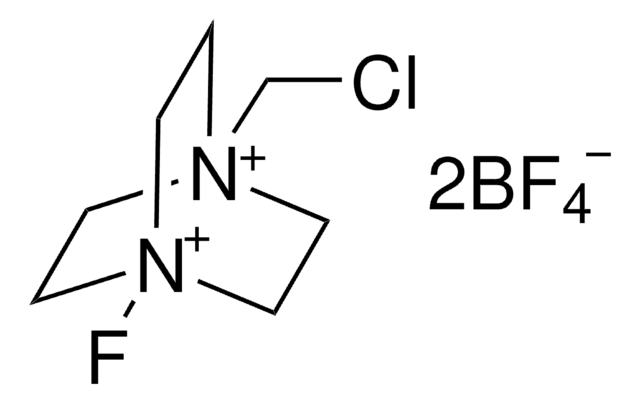

![[Pd(terpy)(MeCN)][BF4]2 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/221/681/ebdc06b7-3b8d-48d4-8aae-08a856260f39/640/ebdc06b7-3b8d-48d4-8aae-08a856260f39.png)
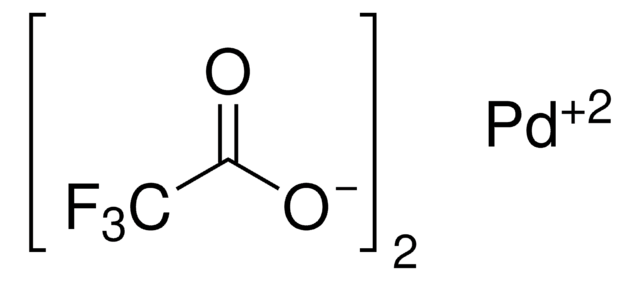
![[1,3-双(二苯基膦)丙烷] 三氟甲磺酸钯 (II)](/deepweb/assets/sigmaaldrich/product/structures/166/337/99b8a24a-ca05-4e6c-81be-76f69b32d1d0/640/99b8a24a-ca05-4e6c-81be-76f69b32d1d0.png)