推薦產品
品質等級
化驗
98%
形狀
solid
bp
113-115 °C/4 mmHg (lit.)
mp
48-50 °C (lit.)
官能基
chloro
ketone
phenyl
SMILES 字串
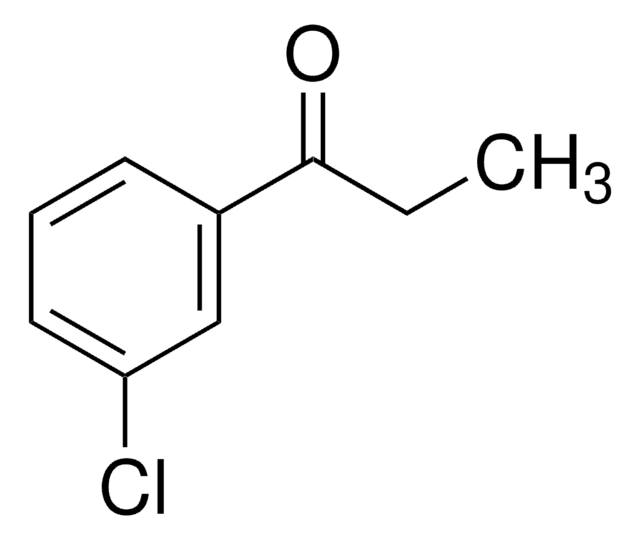
ClCCC(=O)c1ccccc1
InChI
1S/C9H9ClO/c10-7-6-9(11)8-4-2-1-3-5-8/h1-5H,6-7H2
InChI 密鑰
KTJRGPZVSKWRTJ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
3-氯苯丙酮可用于使用固定在藻酸钙凝胶珠中的预热的 产朊假丝酵母 细胞,对(S)-3-氯-1-苯基丙醇进行不对称还原。 利用衍生自(S)-α,α-二苯基脯氨醇的 原位 生成的恶唑硼烷催化剂,它也可通过不对称还原用于合成(R)-3-氯-1-苯基-1-丙醇。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Asymmetric Synthesis of (R)-Fluoxetine: A Practical Approach Using Recyclable and in-situ Generated Oxazaborolidine Catalyst.
Padiya K, et al.
Chin. J. Chem., 27(6), 1137-1140 (2009)
Yang Gen-Sheng et al.
Biotechnology letters, 31(12), 1879-1883 (2009-07-28)
An efficient method for asymmetric reduction of (S)-3-chloro-1-phenylpropanol from 3-chloropropiophenone was developed using preheated Candida utilis cells immobilized in calcium alginate gel beads. Heating the immobilized cells (bead diameter 1.5 mm) at 45 degrees C for 50 min allowed the
Milada Šírová et al.
Journal of drug targeting, 25(9-10), 796-808 (2017-07-21)
Polymer carriers based on N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers with incorporated organic nitrates as nitric oxide (NO) donors were designed with the aim to localise NO generation in solid tumours, thus highly increasing the enhanced permeability and retention (EPR) effect. The NO
Yu-Chang Liu et al.
Organic & biomolecular chemistry, 13(7), 2146-2152 (2014-12-23)
Styrene monooxygenase (SMO) can catalyze the kinetic resolution of secondary allylic alcohols to provide enantiopure glycidol derivatives. To overcome the low theoretical yield of kinetic resolution, we designed a one-pot two-step enzymatic cascade using prochiral α,β-unsaturated ketones as the substrates.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 335614-10G | 4061826740101 |
| 335614-50G | 4061831829433 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務