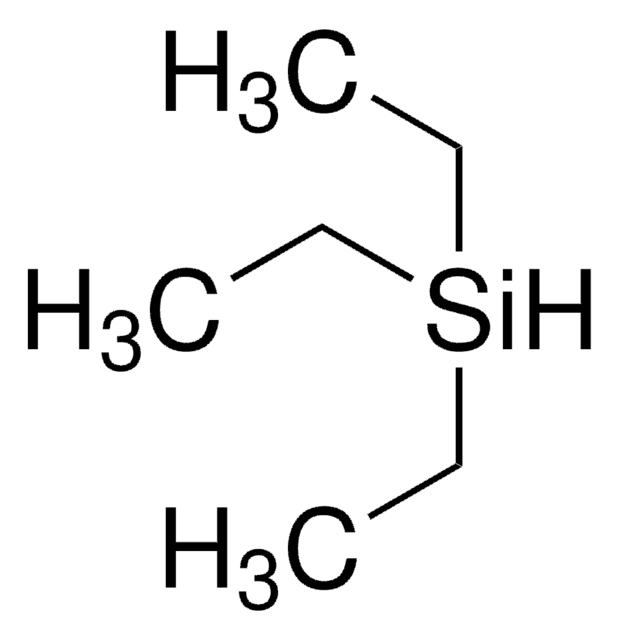
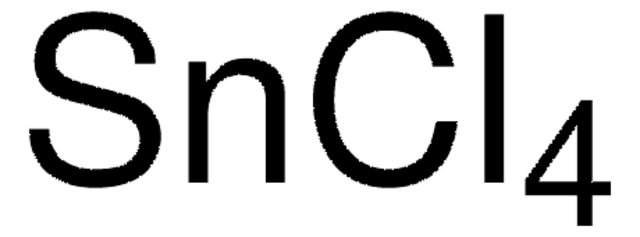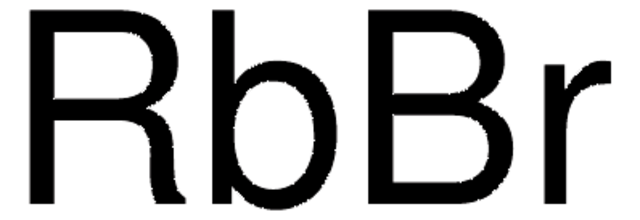推薦產品
化驗
98%
形狀
solid
反應適用性
core: titanium
reagent type: catalyst
bp
230 °C (lit.)
mp
38-40 °C (lit.)
密度
3.25 g/mL at 25 °C (lit.)
SMILES 字串
Br[Ti](Br)(Br)Br
InChI
1S/4BrH.Ti/h4*1H;/q;;;;+4/p-4
InChI 密鑰
UBZYKBZMAMTNKW-UHFFFAOYSA-J
尋找類似的產品? 前往 產品比較指南
應用
溴化钛(IV)(四溴化钛或TiBr4)可作为路易斯酸,用于合成:
- α与芳醛和3-丁炔-2-酮通过贝里斯-希尔曼反应合成α-溴亚甲基羟醛。
- 二溴化钛,其可与铜形成高效的频哪醇反应催化体系。
- 通过炔酰胺和醛或酮反应合成α-溴-γ-羟基烯胺。
特點和優勢
用于在硅基底上形成硅化钛薄膜。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
客戶也查看了
Efficient diastereoselective pinacol reaction of aliphatic and aromatic aldehydes by combined use of newly utilized titanium (II) bromide and copper
Mukaiyama T, et al.
Chemistry Letters (Jpn), 29(4), 336-337 (2000)
Titanium (IV) Bromide and Boron (III) Tribromide Promoted Baylis-Hillman Reactions of Arylaldehydes with But-3-yn-2-one
Shi M and Wang C-J
Helvetica Chimica Acta, 85(3), 841-846 (2002)
Synthesis of α-Halo-γ -hydroxyenamides by Titanium Tetrahalide Mediated Addition of Aldehydes or Ketones to Ynamides
Yabuuchi Y, et al.
Organic Letters, 18(19), 4951-4953 (2016)
K Sellschopp et al.
The Journal of chemical physics, 152(6), 064702-064702 (2020-02-18)
The ability to synthesize nanoparticles (NPs), here TiO2, of different shapes in a controlled and reproducible way is of high significance for a wide range of fields including catalysis and materials design. Different NP shapes exhibit variations of emerging facets
文章
Titanium dioxide (TiO2) is an important n-type semiconducting material that shows interesting characteristics such as photoswitchable surface wettability, high photocatalytic activity, bistable electrical resistance states and high electron drift mobility.
A Review of Mesoporous TiO2 Thin Films
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務