全部照片(1)
About This Item
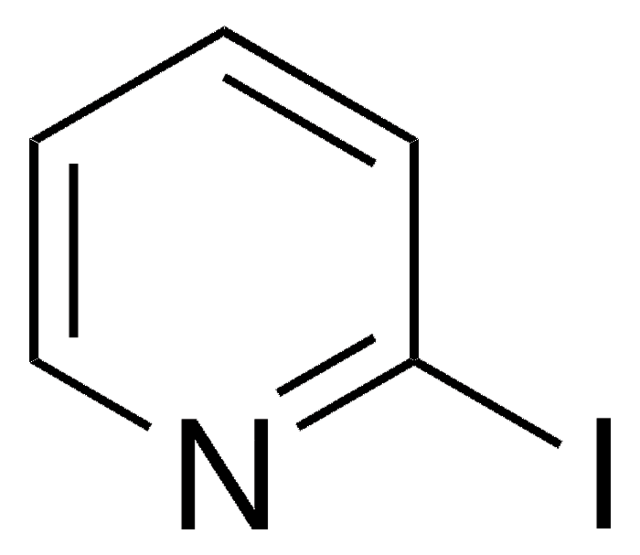
經驗公式(希爾表示法):
C7H6N2
CAS號碼:
分子量::
118.14
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
99%
形狀
liquid
折射率
n20/D 1.626 (lit.)
bp
103 °C/1 mmHg (lit.)
密度
1.165 g/mL at 25 °C (lit.)
SMILES 字串
c1ccn2ccnc2c1
InChI
1S/C7H6N2/c1-2-5-9-6-4-8-7(9)3-1/h1-6H
InChI 密鑰
UTCSSFWDNNEEBH-UHFFFAOYSA-N
一般說明
在 STIB900 小鼠模型中研究了咪唑并 [1,2-a] 吡啶的体内抗锥虫活性。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Suren Husinec et al.
Organic letters, 13(9), 2286-2289 (2011-03-31)
A base promoted cyclization of the protected N-propargylaminopyridines was shown to be an efficient method for the preparation of imidazo[1,2-a]pyridine derivatives. The reactions were carried out with a small excess of base, at room temperature or slightly above producing the
Richard Ducray et al.
Bioorganic & medicinal chemistry letters, 21(16), 4698-4701 (2011-07-22)
We disclose a novel series of insulin-like growth factor-1 receptor kinase inhibitors based on the 3-(pyrimidin-4-yl)-imidazo[1,2-a]pyridine scaffold. The influence on the inhibitory activity of substitution on the imidazopyridine and at the C5 position of the pyrimidine is discussed. In the
Ebrahim Kianmehr et al.
Journal of combinatorial chemistry, 12(1), 41-44 (2009-11-13)
A one-pot, three-component reaction between pyridine, phenacyl bromide, and thiocyanate is described. The reaction afforded the corresponding special type of fully substituted imidazo[1,2-a]pyridine derivatives in good yields without using any catalyst or activation.
Richard Ducray et al.
Bioorganic & medicinal chemistry letters, 21(16), 4702-4704 (2011-07-19)
Following the discovery of imidazopyridine 1 as a potent IGF-1R tyrosine kinase inhibitor, the aniline part has been modified with the aim to optimize the properties of this series. The structure-activity relationships against IGF-1R kinase activity as well as inhibition
Hongpeng Sun et al.
The Journal of organic chemistry, 77(23), 10745-10751 (2012-11-22)
An efficient tandem route to the synthesis of 3H-1,2a(1),3-triazaacenaphthylene derivatives of the cyclazine family has been developed. Target compounds were obtained in moderate to good yields by a Yb(OTf)(3)/Ag(2)CO(3)-catalyzed, three-component domino reaction. This in turn will set the stage for
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![2-phenylimidazo[1,2-a]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/281/247/6c2550a0-2f0c-4866-83d8-3c1fb039e165/640/6c2550a0-2f0c-4866-83d8-3c1fb039e165.png)

![咪唑并[1,2-a]吡嗪 97%](/deepweb/assets/sigmaaldrich/product/structures/370/804/1712d71f-52fb-4758-9a22-85b6c96cd4e8/640/1712d71f-52fb-4758-9a22-85b6c96cd4e8.png)
![Imidazo[1,2-a]pyrimidine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/187/001/4862c14e-bec7-4475-85a5-f178e48ff60f/640/4862c14e-bec7-4475-85a5-f178e48ff60f.png)