推薦產品
品質等級
化驗
98%
mp
53-56 °C (lit.)
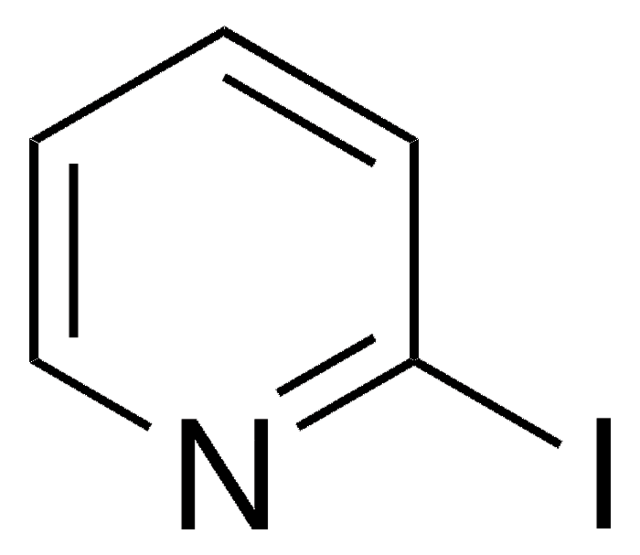
SMILES 字串
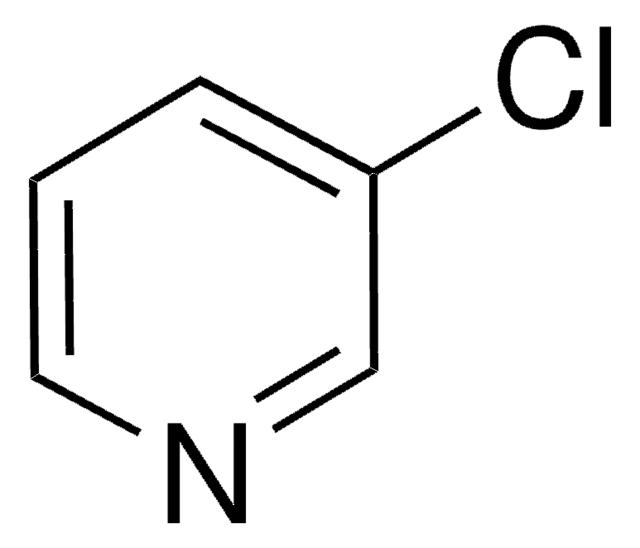
Ic1cccnc1
InChI
1S/C5H4IN/c6-5-2-1-3-7-4-5/h1-4H
InChI 密鑰
XDELKSRGBLWMBA-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
3-Iodopyridine is a heteroaryl halide. It undergoes microwave-assisted coupling with heterocyclic compounds (pyrazole, imidazole, pyrrole and indole) to afford the corresponding N-3-pyridinyl-substituted heterocyclic compounds.
應用
3-Iodopyridine may be used to synthesize following pyridine alkaloids:
- theonelladins C
- theonelladins D
- niphatesine C
- xestamine D
訊號詞
Warning
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
224.1 °F - closed cup
閃點(°C)
106.7 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Microwave-assisted solvent-and ligand-free copper-catalysed cross-coupling between halopyridines and nitrogen nucleophiles.
Ernst, R. F.
Green Chemistry, 13(1), 42-45 (2011)
Synthesis of pyridine alkaloids via Pd-catalyzed coupling of 3-iodopyridine, 1, ?-dienes and nitrogen nucleophiles.
Larock RC and Wang Y.
Tetrahedron Letters, 43(1), 21-23 (2002)
Alina K Feldman et al.
Organic letters, 6(22), 3897-3899 (2004-10-22)
[reaction: see text] 1,4-Disubstituted 1,2,3-triazoles are obtained in excellent yields by a convenient one-pot procedure from a variety of readily available aromatic and aliphatic halides without isolation of potentially unstable organic azide intermediates.
Cameron C Bright et al.
Physical chemistry chemical physics : PCCP, 19(46), 31072-31084 (2017-11-21)
Small nitrogen containing heteroaromatics are fundamental building blocks for many biological molecules, including the DNA nucleotides. Pyridine, as a prototypical N-heteroaromatic, has been implicated in the chemical evolution of many extraterrestrial environments, including the atmosphere of Titan. This paper reports
Patrick W Fedick et al.
Journal of the American Society for Mass Spectrometry, 30(10), 2144-2151 (2019-08-09)
Suzuki cross-coupling is a widely performed reaction, typically using metal catalysts under heated conditions. Acceleration of the Suzuki cross-coupling reaction has been previously explored in microdroplets using desorption electrospray ionization mass spectrometry (DESI-MS). Building upon previous work, presented here is
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務