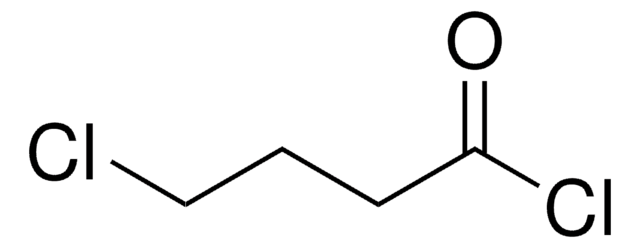
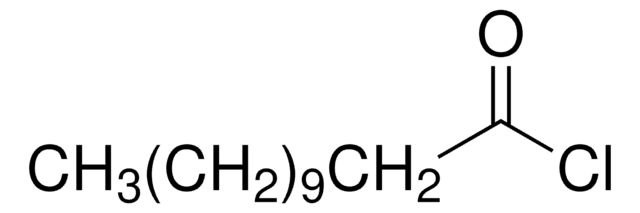推薦產品
蒸汽密度
3.7 (vs air)
品質等級
化驗
≥99%
形狀
liquid
折射率
n20/D 1.412 (lit.)
bp
102 °C (lit.)
mp
−89 °C (lit.)
溶解度
diethyl ether: miscible(lit.)
密度
1.026 g/mL at 25 °C (lit.)
官能基
acyl chloride
SMILES 字串
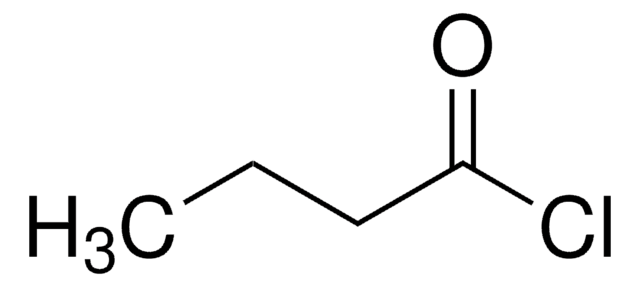
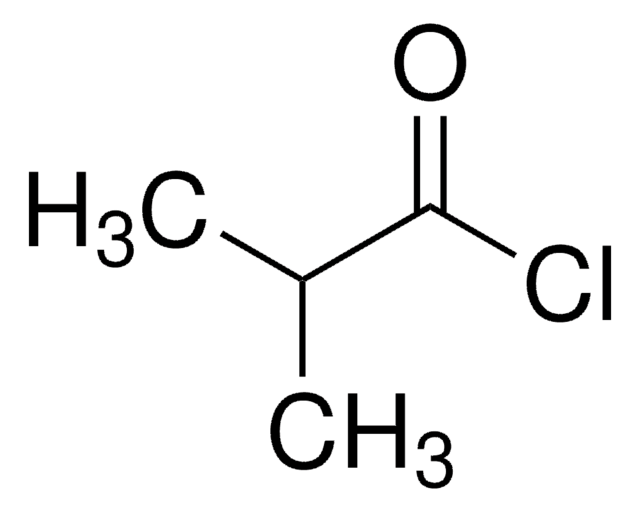
CCCC(Cl)=O
InChI
1S/C4H7ClO/c1-2-3-4(5)6/h2-3H2,1H3
InChI 密鑰
DVECBJCOGJRVPX-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Photchlorination of butyryl chloride in liquid phase has been investigated.
應用
Butyryl chloride can be used as:
- A reagent in the acylation of thiophene in the liquid phase catalyzed by zeolites.
- A source of CO and -Cl in the chlorocarbonylation of aryl bromides to yield acid chlorides.
- An intermediate in the synthesis of aryl [11C]methylsulfones for PET applications.
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
64.4 °F - DIN 51755 Part 1
閃點(°C)
18 °C - DIN 51755 Part 1
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
A lewis acid site-activated reaction in zeolites: thiophene acylation by butyryl chloride.
Isaev Y and Fripiat JJ.
J. Catal., 182(1), 257-263 (1999)
CHLORINATION OF BUTYRYL CHLORIDE IN THE LIQUID-PHASE.
Korhonen IO.
Acta Chemica Scandinavica. Series B, 35(3), 175-178 (1981)
A general method for the synthesis of aryl [11C] methylsulfones: potential PET probes for imaging cyclooxygenase-2 expression
Majo VJ, et al.
Bioorganic & Medicinal Chemistry Letters, 15(19), 4268-4271 (2005)
Aleksandra Leśniarek et al.
Molecules (Basel, Switzerland), 25(5) (2020-03-04)
The influence of buffer type, co-solvent type, and acyl chain length was investigated for the enantioselective hydrolysis of racemic 4-arylbut-3-en-2-yl esters using Lecitase™ Ultra (LU). Immobilized preparations of the Lecitase™ Ultra enzyme had significantly higher activity and enantioselectivity than the
Paulina Majewska
Bioorganic chemistry, 61, 28-35 (2015-06-13)
2-Hydroxy-2-(ethoxyphenylphosphinyl)acetic acid, a new type of organophosphorus compound possessing two stereogenic centers, was investigated. Racemic 2-butyryloxy-2-(ethoxyphenylphosphinyl)acetic acid was synthesized and hydrolyzed using four bacterial species as biocatalysts. In all cases the reaction was more or less stereoselective and isomers bearing
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務