全部照片(1)
About This Item
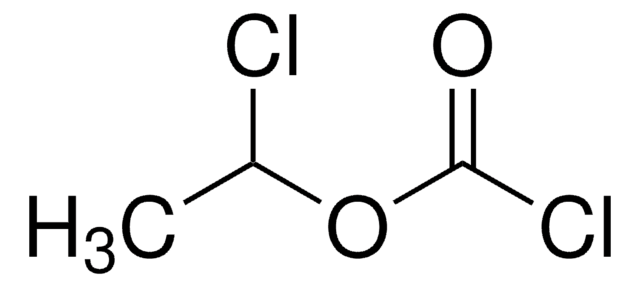
線性公式:
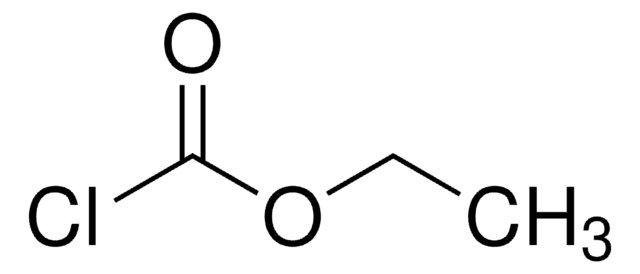
ClCOOCH2Cl
CAS號碼:
分子量::
128.94
Beilstein:
506426
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
≥98.0% (GC)
形狀
liquid
折射率
n20/D 1.428
bp
107-108 °C (lit.)
密度
1.450 g/mL at 20 °C
官能基
chloro
儲存溫度
2-8°C
SMILES 字串
ClCOC(Cl)=O
InChI
1S/C2H2Cl2O2/c3-1-6-2(4)5/h1H2
InChI 密鑰
JYWJULGYGOLCGW-UHFFFAOYSA-N
應用
氯甲酸氯甲基酯作为关键反应物用于合成:
- 双氯芬酸和氟芬那酸的新型氨基羰基氧甲酯
- 环孢菌素 A 的高水溶性单甲氧基聚乙二醇前体药物
- 3-乙酰氧基甲氧羰基-1-芳基-3-甲基三氮烯系列
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Inhalation - Skin Corr. 1B
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
203.0 °F - closed cup
閃點(°C)
95 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Hoon Cho et al.
Archives of pharmacal research, 27(6), 662-669 (2004-07-31)
The highly water-soluble monomethoxypoly(ethyleneglycol) (mPEG) prodrugs of cyclosporin A (CsA) were synthesized. These prodrugs were prepared by initially preparing intermediate in the form of carbonate at the 3'-positions of CsA with chloromethyl chloroformate, in the presence of a base to
Lina Ribeiro et al.
Archiv der Pharmazie, 340(1), 32-40 (2007-01-09)
Aminocarbonyloxymethyl ester prodrugs are known to undergo rearrangement in aqueous solutions to form the corresponding N-acylamine side product via an O-->N intramolecular acyl transfer from the carbamate conjugate base. Novel aminocarbonyloxymethyl esters of diclofenac and flufenamic acid containing amino acid
Keivan Sadrerafi et al.
Drug design, development and therapy, 12, 987-995 (2018-05-08)
Our previous study indicated that carborane containing small-molecule 1-(hydroxymethyl)-7-(4'-(trans-3″-(3'″-pyridyl)acrylamido)butyl)-1,7-dicarbadodecaborane (hm-MC4-PPEA), was a potent inhibitor of nicotinamide phosphoribosyltransferase (Nampt). Nampt has been shown to be upregulated in most cancers and is a promising target for the treatment of many different types
E Carvalho et al.
Bioorganic & medicinal chemistry, 8(7), 1719-1725 (2000-09-08)
A series of 3-acyloxymethyloxycarbonyl-1-aryl-3-methyltriazenes 5 was synthesised by the sequential reaction of 1-aryl-3-methyltriazenes with (i) chloromethyl chloroformate, (ii) NaI in dry acetone, and (iii) either the silver carboxylate or the carboxylic acids in the presence of silver carbonate. The hydrolysis
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務