About This Item
推薦產品
品質等級
化驗
97%
形狀
powder and chunks
反應適用性
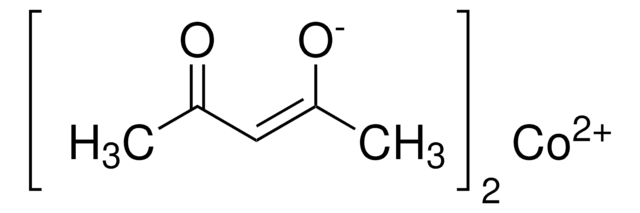
core: cobalt
雜質
≤3% water
mp
165-170 °C (lit.)
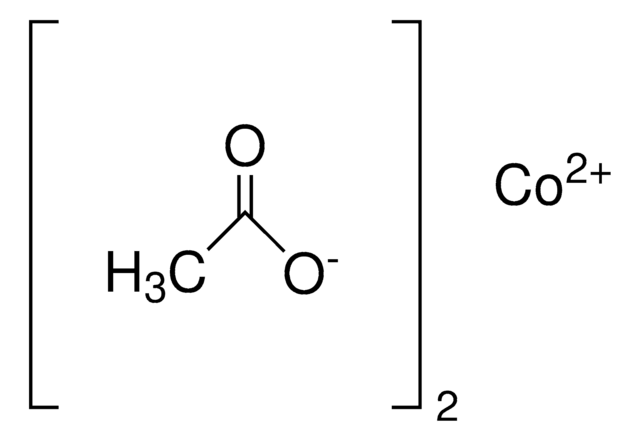
SMILES 字串
CC(=O)\C=C(\C)O[Co]O\C(C)=C/C(C)=O
InChI
1S/2C5H8O2.Co/c2*1-4(6)3-5(2)7;/h2*3,6H,1-2H3;/q;;+2/p-2/b2*4-3-;
InChI 密鑰
UTYYEGLZLFAFDI-FDGPNNRMSA-L
尋找類似的產品? 前往 產品比較指南
一般說明
應用
- 钴(II)催化的异氰化物与胺的插入反应:详细介绍了一种由乙酰丙酮钴(II)催化形成脲和氮杂杂环化合物的合成方法,该方法适用于药物合成(Zhu et al., 2014)。
- 亚氨酸酯辅助下钴催化的C-H官能化:描述了一种使用乙酰丙酮钴(II)进行C-H官能化的方法,该方法对有机合成和材料化学很重要(Mei & Ackermann, 2016)。
- 乙酰丙酮钴(II)在磁性介孔二氧化硅纳米球上的共价锚定:重点介绍了其作为烯烃环氧化反应催化剂的用途,该研究与催化研究相关(Li et al., 2015)。
- 溶剂热合成Co3O4纳米颗粒的前体。这些纳米颗粒表现出很高的电化学性能,由于其优异的电容和循环稳定性,被用作潜在的超级电容器材料。
- 水热法制备Co3O4纳米颗粒的前体。所得的Co3O4纳米颗粒具有高度均匀的介孔结构和可调的尺寸,使其在CO传感应用中具有前景。
- 利用金属有机化学气相沉积(MOCVD)制备氧化钴薄膜的前体。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
文章
Magnetism and magnetic materials have been of scientific interest for over 1,000 years. More recently, fundamental investigations have focused on exploring the various types of magnetic materials and understanding the magnetic effects created by electric currents.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 227129-250G | 4061838780607 |
| 227129-50G | 4061838780614 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務