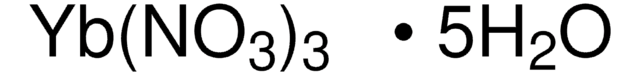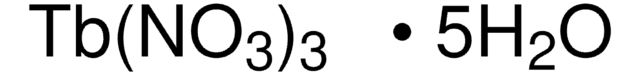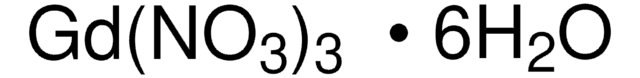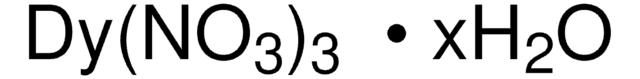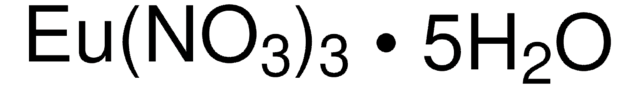About This Item
推薦產品
化驗
99.999% trace metals basis
形狀
crystals and lumps
反應適用性
reagent type: catalyst
core: cerium
雜質
≤15.0 ppm Trace Metal Analysis
SMILES 字串
[Ce+3].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Ce.3NO3.6H2O/c;3*2-1(3)4;;;;;;/h;;;;6*1H2/q+3;3*-1;;;;;;
InChI 密鑰
QQZMWMKOWKGPQY-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
訊號詞
Danger
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
儲存類別代碼
5.1B - Oxidizing hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
文章
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務