推薦產品
品質等級
化驗
96%
形狀
solid
mp
39-45 °C (lit.)
官能基
hydroxyl
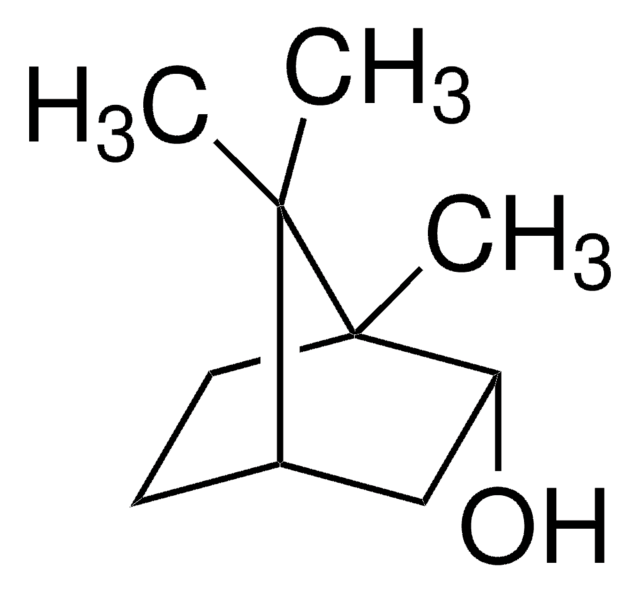
SMILES 字串
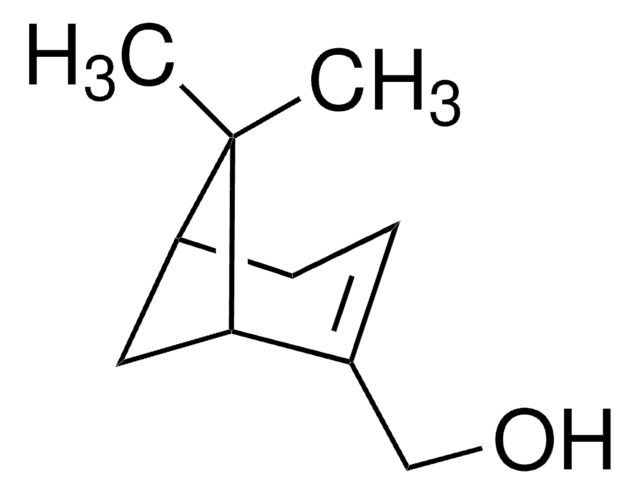
CC1(C)[C@H]2CC[C@](C)(C2)[C@H]1O
InChI
1S/C10H18O/c1-9(2)7-4-5-10(3,6-7)8(9)11/h7-8,11H,4-6H2,1-3H3/t7-,8-,10+/m0/s1
InChI 密鑰
IAIHUHQCLTYTSF-OYNCUSHFSA-N
一般說明
(1R)-endo-(+)-葑基醇是包含葑烷骨架的双环单萜。它是香料和调味剂中常用的挥发性化合物。
應用
(1R)-endo-(+)-葑基醇可用于制备其他萜类化合物,例如(+)-(1R,2S)-10-羟基葑醇,(+)-(1R,2R,3S)-8-羟基葑醇 ,(1S,2S,6S)-6-外羟基葑醇和(-)-(1R,2R,3R)-9-羟基葑醇。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
165.2 °F - closed cup
閃點(°C)
74 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
M Miyazawa et al.
Xenobiotica; the fate of foreign compounds in biological systems, 37(9), 943-953 (2007-11-10)
The metabolism of (+)-fenchol was investigated in vitro using liver microsomes of rats and humans and recombinant cytochrome P450 (P450 or CYP) enzymes in insect cells in which human/rat P450 and NADPH-P450 reductase cDNAs had been introduced. The biotransformation of
Sergio Abbate et al.
Chirality, 21 Suppl 1, E242-E252 (2009-11-21)
The first well documented experiments of Near Infrared Vibrational Circular Dichroism (NIR-VCD) were performed around 1975. We review the thirty year history of NIR-VCD, encompassing both instrumental development and theoretical/computational methods that allow interpretation of experimental spectra, harvesting useful structural
D M Satterwhite et al.
The Journal of biological chemistry, 260(26), 13901-13908 (1985-11-15)
The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of this bound intermediate. To test this stereochemical scheme, phosphatase-free preparations of (-)-endo-fenchol cyclase from
D Yang et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 22(3), 128-131 (2003-02-11)
The essential oil isolated from the dried leaves of Lindera communis was analyzed by means of gas chromatography-mass(GC-MS) technique, the structures of 23 chemical components were identified from it in total, among these, (-)-spathulenol(relative content 22.50%), endo-1,3,3-trimethyl-2-norbornanol, acetate (10.06%), caryophyllene
R Croteau et al.
The Journal of biological chemistry, 263(30), 15449-15453 (1988-10-25)
The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of this bound intermediate. Incubation of (1R)-[2-14C,1-3H]- and (1S)-[2-14C,1-3H]geranyl pyrophosphate with a preparation of (-)-endo-fenchol
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務