全部照片(1)
About This Item
經驗公式(希爾表示法):
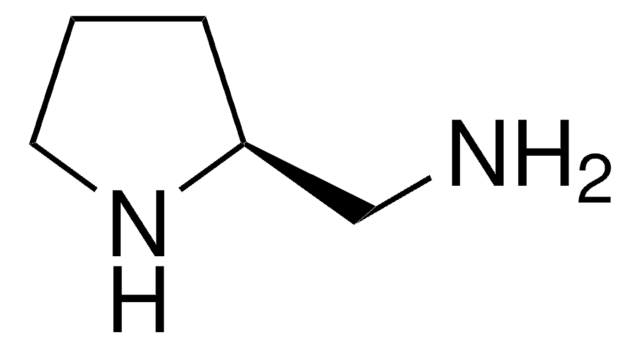
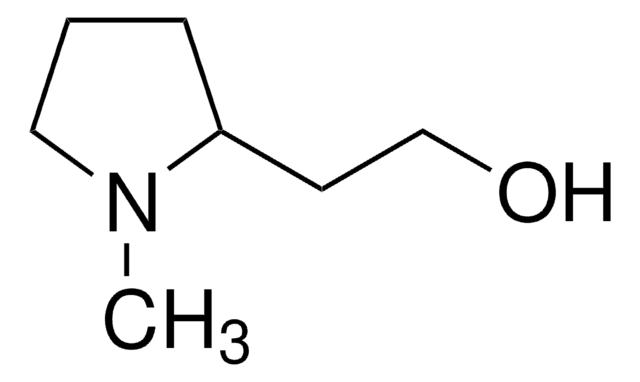
C5H11NO
CAS號碼:
分子量::
101.15
Beilstein:
79843
EC號碼:
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
光學活性
[α]20/D +31°, c = 1 in toluene
折射率
n20/D 1.4853 (lit.)
bp
74-76 °C/2 mmHg (lit.)
密度
1.025 g/mL at 25 °C (lit.)
官能基
hydroxyl
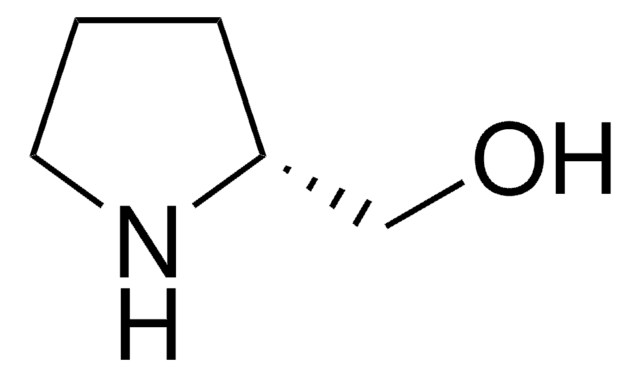
SMILES 字串
OC[C@@H]1CCCN1
InChI
1S/C5H11NO/c7-4-5-2-1-3-6-5/h5-7H,1-4H2/t5-/m0/s1
InChI 密鑰
HVVNJUAVDAZWCB-YFKPBYRVSA-N
一般說明
(S)-(+)-2-吡咯烷基甲醇又称为(S)-(+)-脯氨醇,在手性有机物合成中用作手性合成砌块。还可用作不对称合成的手性助剂和不对称催化的手性配体。
應用
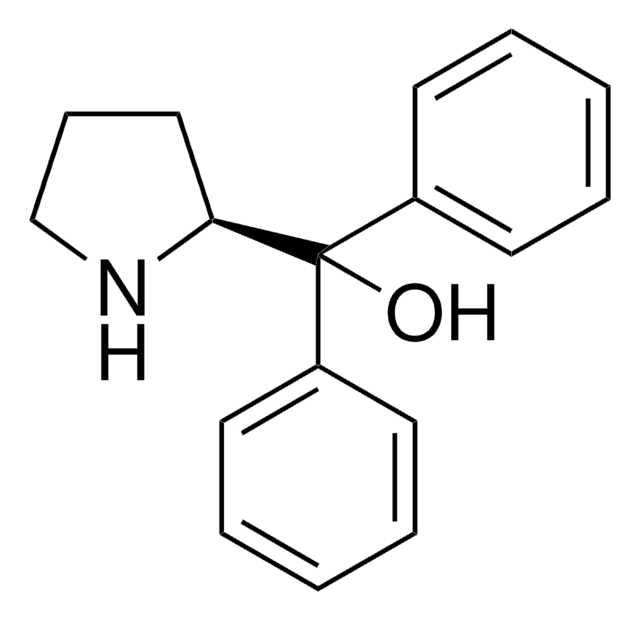
(S)-(+)-2-吡咯烷基甲醇可作为起始原料合成(S)-α,α-二芳基-2-吡咯烷基甲醇。在合成中起着赋予终产物手性的重要作用。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
186.8 °F - closed cup
閃點(°C)
86 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Yuko Kawanami et al.
The Journal of organic chemistry, 74(20), 7908-7921 (2009-09-19)
The photochirogenesis of 2-anthracenecarboxylic acid (AC) complexed to a hydrogen-bonding template (TKS159) was investigated to obtain mechanistic information on how chirogenesis is achieved for the dimerization of AC. Complexation of AC to TKS159 leads to the shielding of one of
Satnam Lidder et al.
Journal of medical toxicology : official journal of the American College of Medical Toxicology, 4(3), 167-169 (2008-09-30)
Many countries have specific legislation, such as the Controlled Substances Act (1970) in the United States and the Misuse of Drugs Act (1971) in the United Kingdom to control recreational drugs. There is a growing market and supply of "novel"
Patrick Bolze et al.
Organic letters, 10(17), 3753-3756 (2008-07-29)
A simple organocatalytic approach to highly attractive chiral building blocks is presented. By the reaction of beta-ketoesters with alpha,beta-unsaturated aldehydes using a chiral TMS-protected prolinol as the catalyst, optically active 5-(trialkylsilyl)cyclohex-2-enones are formed in good yields and with 98-99% ee.
Magnus Rueping et al.
Organic & biomolecular chemistry, 10(30), 6201-6210 (2012-05-16)
A highly efficient route for the synthesis of valuable 3,4-substituted chromenone derivatives by the reaction of 1,3-diketones with aldehydes in the presence of l-proline was developed. The reactions take advantage of readily available starting materials and follow a Knoevenagel condensation/Michael
Tatsuya Urushima et al.
Organic letters, 12(13), 2966-2969 (2010-06-10)
Diarylprolinol was found to be an effective organocatalyst of the direct, enantioselective aldol reaction of commercially available polymeric ethyl glyoxylate, affording gamma-ethoxycarbonyl-beta-hydroxy aldehydes, versatile synthetic intermediates, in good yield with excellent enantioselectivity.
Global Trade Item Number
| 庫存單位 | GTIN |
|---|---|
| 186511-5G | 4061838758187 |
| 186511-1G | 4061838096388 |
| 186511-25G | 4061838118417 |
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務