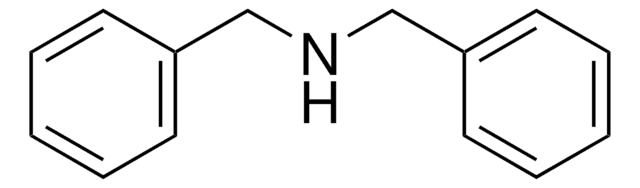
推薦產品
品質等級
化驗
≥99%
形狀
solid
bp
306-307 °C (lit.)
mp
35-38 °C (lit.)
溶解度
alcohol: soluble
chloroform: soluble
diethyl ether: soluble
water: insoluble
密度
1.061 g/mL at 25 °C (lit.)
官能基
amine
phenyl
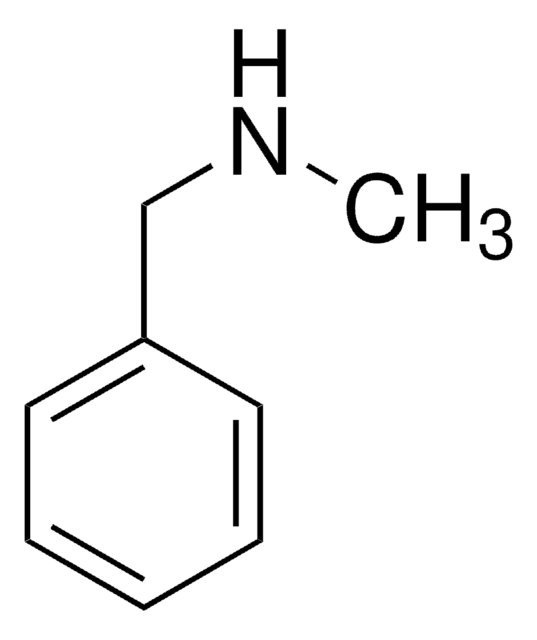
SMILES 字串
C(Nc1ccccc1)c2ccccc2
InChI
1S/C13H13N/c1-3-7-12(8-4-1)11-14-13-9-5-2-6-10-13/h1-10,14H,11H2
InChI 密鑰
GTWJETSWSUWSEJ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
The electropolymerisation of N-benzylaniline at transparent Indium Tin Oxide glass electrodes has been investigated by UV-visible spectroelectrochemistry. N-Benzylaniline on electrochemical oxidation in aqueous sulfuric acid solution produces an adherent conducting polymer film at the platinum electrode.
應用
N-Benzylaniline was used in the separation of tervalent gallium, indium and thallium by solvent extraction method.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
M M Khosla et al.
Talanta, 21(6), 411-415 (1974-06-01)
A simple and rapid method is proposed for the separation of tervalent gallium, indium and thallium by solvent extraction with N-benzylaniline in chloroform from different concentrations of hydrochloric acid. Thallium and gallium are extracted from 1M and 7.0-7.5M hydrochloric acid
A UV-visible spectroelectrochemical study of the electropolymerisation of N-benzylaniline.
Malinauskas A and Holze R.
Journal of Solid State Electrochemistry, 3(7-8), 429-436 (1999)
Electrochemical polymerization and characterization of N-benzylaniline.
Dong S and Li Z.
Synthetic Metals, 33(1), 93-98 (1989)
Synthesis and structure-activity relationships of a class of sodium iodide symporter function inhibitors.
Fanny Waltz et al.
ChemMedChem, 6(10), 1775-1777 (2011-07-14)
M Ulgen et al.
Xenobiotica; the fate of foreign compounds in biological systems, 24(8), 735-748 (1994-08-01)
1. The in vitro hepatic microsomal metabolism of certain substituted N-benzylanilines was studied in the male hamster to establish the mechanism(s) and process(es) involved in the formation of the corresponding amides. 2. N-Benzyl-2,4,6-trihalogeno, N-benzyl-4-cyano- and N-benzyl-4-nitroanilines were only metabolized by
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務