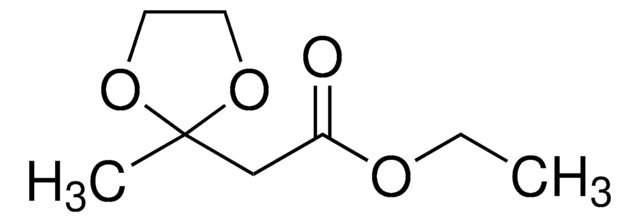
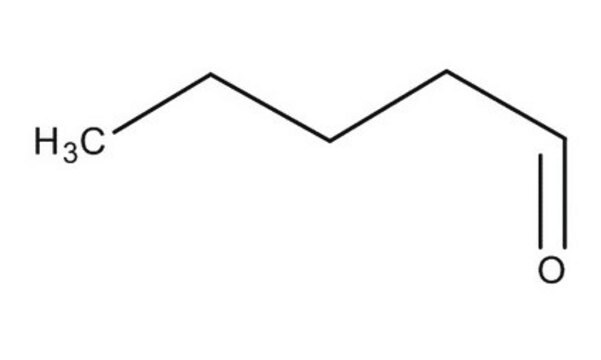
推薦產品
一般說明
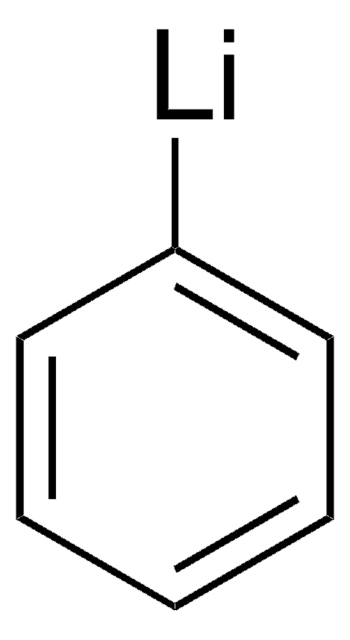
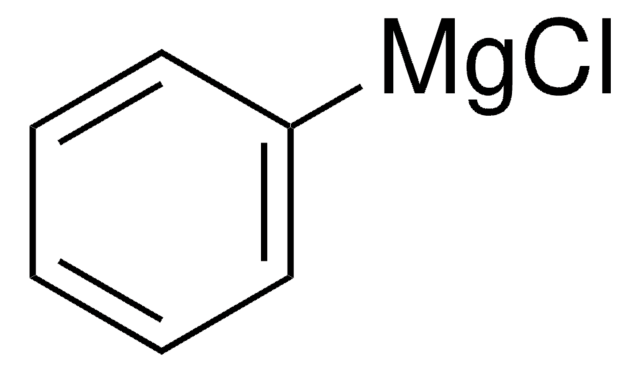
Phenylmagnesium bromide solution contains 3M phenylmagnesium bromide in diethyl ether. It can act as a strong acid and Lewis acid. It can undergo addition reaction with many unsaturated functional groups. The phenyl group can displace halide from other organic compounds. Phenylmagnesium bromide is a Grignard reagent. Reaction of β-cyclohexanedione (dihydroresorcinol) with phenylmagnesium bromide has been investigated.
應用
Phenylmagnesium bromide was used for the synthesis of end-functionalized regioregular poly(3-alkylthiophene)s. It was also used for the monoalkylation of aliphatic primary amine to generate secondary amines by the Grignard reaction of 1-[(alkylamino) methyl] benzotriazoles.
It may be used for synthesis of the following:
It may be used for synthesis of the following:
- 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol
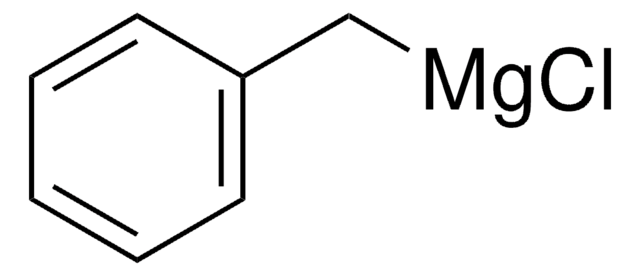
- 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol
- (3-(2-Dithiobenzoatepropionyl)propyl)dimethylmethoxysilane, reversible addition-fragmentation chain transfer polymerization (RAFT)-silane agent
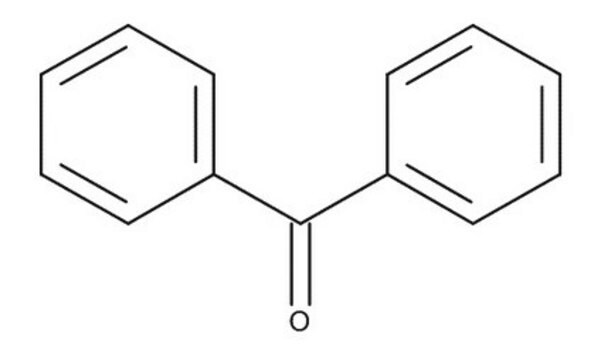
- series of o-substituted benzophenones
包裝
其他說明
储存温度低于 25°C 可能形成结晶镁盐。将容器移至温暖处不时温和搅拌可使固体重新溶解
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
-40.0 °F - closed cup
閃點(°C)
-40 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
客戶也查看了
Synthesis of O-substituted benzophenones by Grignard reaction of 3-substituted isocoumarins.
Manivel P, et al.
Journal of the Chilean Chemical Society, 53(3), 1609-1610 (2008)
The reaction of beta-cyclohexanedione (dihydroresorcinol) and its ethyl enol ether with phenylmagnesium bromide.
G F WOODS et al.
Journal of the American Chemical Society, 70(6), 2174-2177 (1948-06-01)
Synthesis of well-defined polymer brushes grafted onto silica nanoparticles via surface reversible addition-fragmentation chain transfer polymerization.
Li C and Benicewicz BC.
Macromolecules, 38(14), 5929-5936 (2005)
S Schenone et al.
Farmaco (Societa chimica italiana : 1989), 45(12), 1309-1325 (1990-12-01)
The synthesis of 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol 2 and 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol 3 starting from (+)-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-one and phenylmagnesium bromide or benzylmagnesium chloride, respectively, is described. Alcohols 2 and 3 gave a series of omega-dialkylaminoalkyl ethers 4 by reaction as sodium salts with omega-chloroalkyldialkylamines in toluene
Phenylmagnesium Bromide.
Richey HG, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2009)
文章
Reagents for C–C Bond Formation
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務