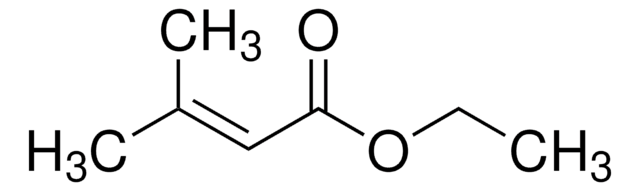
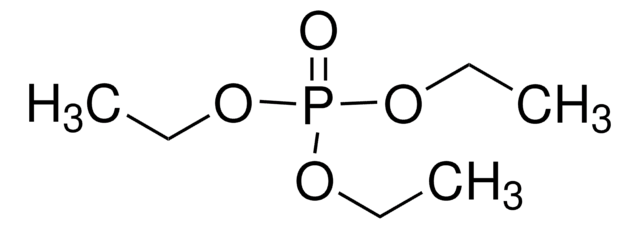
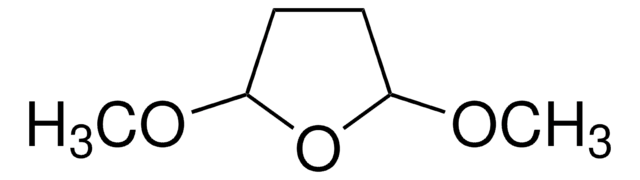
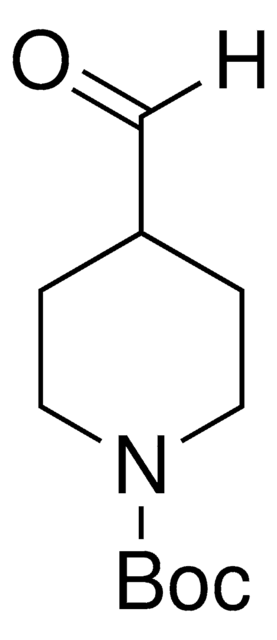
推薦產品
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.479 (lit.)
bp
92-94 °C/15 mmHg (lit.)
密度
1.04 g/mL at 25 °C (lit.)
SMILES 字串
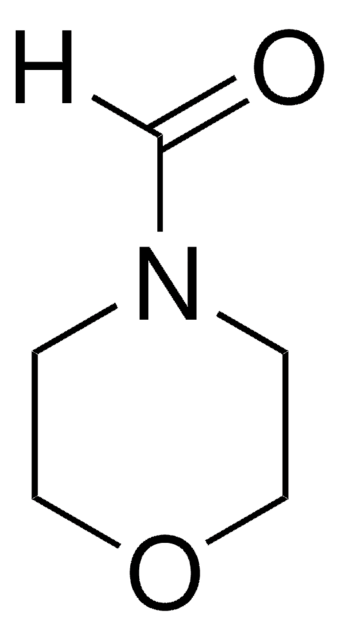
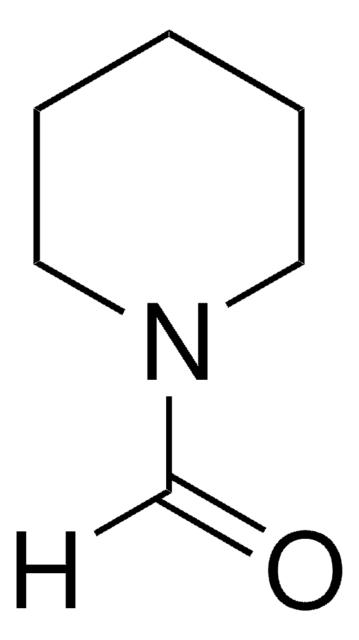
[H]C(=O)N1CCCC1
InChI
1S/C5H9NO/c7-5-6-3-1-2-4-6/h5H,1-4H2
InChI 密鑰
AGRIQBHIKABLPJ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
1-Formylpyrrolidine is the monomer constituent of gas clathrate inhibitor.
應用
1-Formylpyrrolidine was used in the synthesis of 1-oxa-3,4-dimethyl-5-(1-pyrrolldino)-2,2-di(tert-butyl)silacyclopentane and 1-oxa-4-isopropyl-5-(1-pyrrolidino)-2,2-di(tert-butyl)silacyclopentane.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
203.0 °F - closed cup
閃點(°C)
95 °C - closed cup
個人防護裝備
Eyeshields, Gloves
客戶也查看了
Preparation and synthetic utility of oxasilacyclopentane acetals derived from siliranes.
Shaw JT and Woerpel KA.
Tetrahedron, 53(48), 16597-16606 (1997)
Computational studies of structure and dynamics of clathrate inhibitor monomers in solution.
Gomez G, et al.
Industrial & Engineering Chemistry Research, 46(1), 131-142 (2007)
Joseph Bejjani et al.
The Journal of organic chemistry, 68(25), 9747-9752 (2003-12-06)
N-Tritylprolinal (prepared in four steps from l-proline) shows a very high Felkin diastereoselectivity in its reaction with various nucleophiles, leading to a straightforward and highly stereoselective access to syn-proline-derived amino alcohols.
T Yoshimoto et al.
Journal of biochemistry, 98(4), 975-979 (1985-10-01)
The inhibitory effects of proline-containing peptides and their derivatives on prolyl endopeptidases from Flavobacterium meningosepticum and bovine brain were compared. Replacement of the carboxyl terminal proline in N-blocked peptides with prolinal resulted in remarkable decreases in Ki values for both
M Saito et al.
Journal of enzyme inhibition, 3(3), 163-178 (1990-01-01)
Several prolinal derivatives were synthesized and examined for their inhibitory activity on post-proline cleaving enzymes from Flavobacterium meningosepticum and bovine brain and their possible properties as nootropic agents. Almost all the compounds tested inhibited the activity of both enzymes at
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務