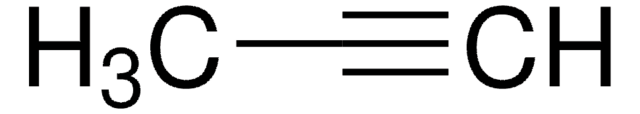推薦產品
化驗
99%
形狀
liquid
折射率
n20/D 1.564 (lit.)
bp
185 °C (lit.)
密度
0.928 g/mL at 25 °C (lit.)
官能基
phenyl
儲存溫度
2-8°C
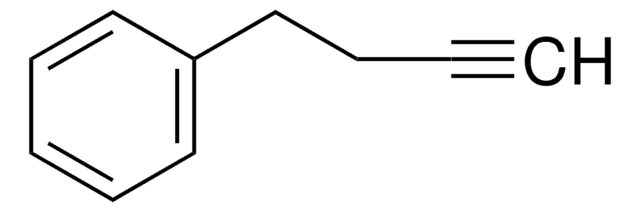
SMILES 字串
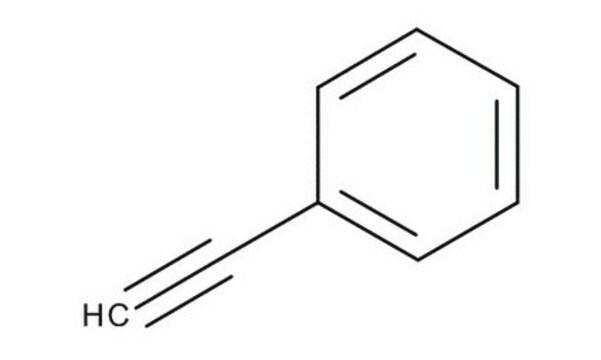
CC#Cc1ccccc1
InChI
1S/C9H8/c1-2-6-9-7-4-3-5-8-9/h3-5,7-8H,1H3
InChI 密鑰
GHUURDQYRGVEHX-UHFFFAOYSA-N
一般說明
研究人员已经报道过环戊烯和1-苯基-1-丙炔的共催化反应。研究人员采用程序升温脱附(temperature-programmed desorption)、X射线和双光子发射光谱研究了100K 下铜(111)上1-苯基-1-丙炔的成键性质。1-苯基-1-丙炔是多巴胺-β羟化酶的抑制剂。研究人员已经报道过通过TaCl5和 NbCl5进行1-苯基-1-丙炔的聚合。
1-苯基-1-丙炔可作为各种化学反应的原料,如加成反应和聚合。
1-苯基-1-丙炔可作为各种化学反应的原料,如加成反应和聚合。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
143.6 °F - closed cup
閃點(°C)
62 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Youngku Sohn et al.
Langmuir : the ACS journal of surfaces and colloids, 23(24), 12185-12191 (2007-10-31)
The bonding properties of 1-phenyl-1-propyne (PP, C6H5CCCH3) on Cu(111) at 100 K have been studied using temperature-programmed desorption (TPD), and X-ray, ultraviolet, and two-photon photoemission spectroscopies (XPS, UPS, and 2PPE). In TPD, there is no evidence for dissociation. Multilayer desorption
Effect of organometallic cocatalysts on the polymerization of 1-phenyl-1-propyne by tantalum pentachloride (TaCl5) and niobium pentachloride (NbCl5).
Masuda T, et al.
Macromolecules, 18(11), 2109-2113 (1985)
Shuhuai Xiang et al.
Journal of the American Chemical Society, 136(16), 5832-5835 (2014-04-12)
Here we report a general method for the measurement of (13)C kinetic isotope effects at natural abundance for reactions that yield two or more products concurrently. We use, as an example, a recently reported Co-catalyzed reaction between cyclopentene and 1-phenyl-1-propyne.
Ekaterina V Pokochueva et al.
Physical chemistry chemical physics : PCCP, 21(48), 26477-26482 (2019-11-30)
Parahydrogen-induced polarization (PHIP) is a powerful technique for studying hydrogenation reactions in gas and liquid phases. Pairwise addition of parahydrogen to the hydrogenation substrate imparts nuclear spin order to reaction products, manifested as enhanced 1H NMR signals from the nascent
G Colombo et al.
The Journal of biological chemistry, 259(24), 15017-15020 (1984-12-25)
The catalytic action of dopamine beta-hydroxylase on 1-phenyl-1-propyne results in concomitant loss of enzyme activity. At pH 5.5 and 25 degrees C, 1-phenyl-1-propyne inactivates dopamine beta-hydroxylase in a mechanism-based fashion. The inactivation rate is first-order, follows saturation kinetics, and is
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務