全部照片(2)
About This Item
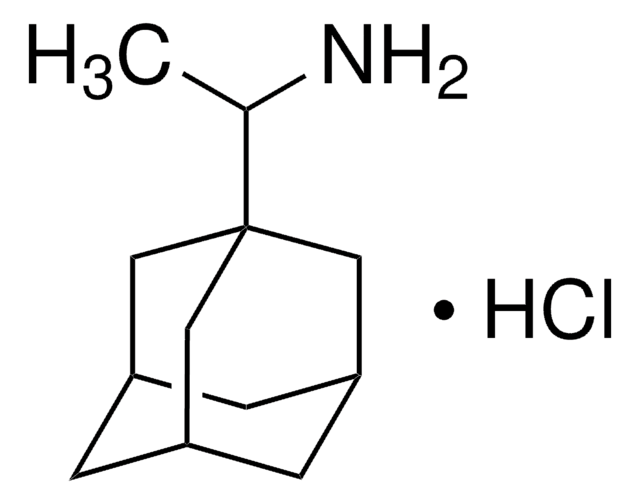
經驗公式(希爾表示法):
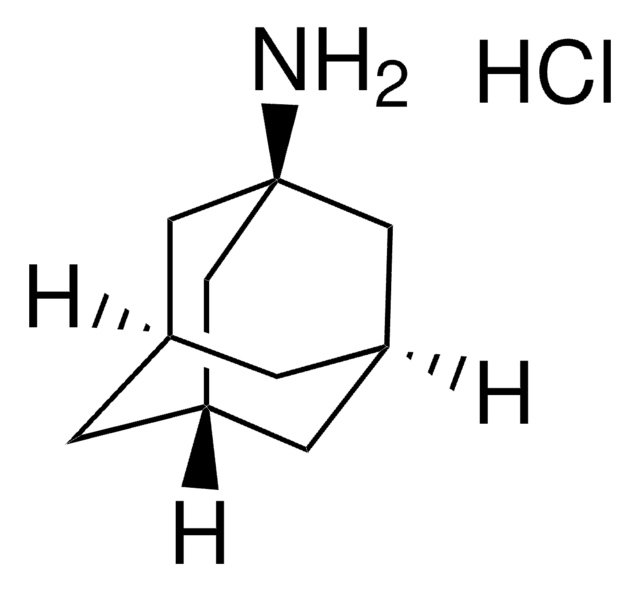
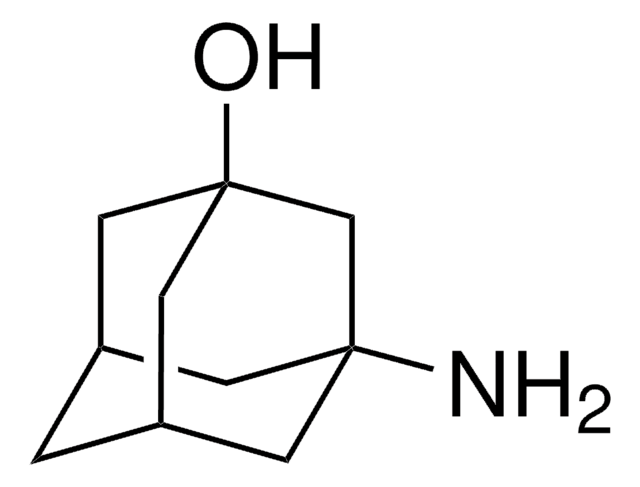
C10H17N · HCl
CAS號碼:
分子量::
187.71
Beilstein:
4297901
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
99%
形狀
solid
mp
>300 °C (lit.)
SMILES 字串
Cl.NC1[C@@H]2C[C@H]3C[C@@H](C2)C[C@@H]1C3
InChI
1S/C10H17N.ClH/c11-10-8-2-6-1-7(4-8)5-9(10)3-6;/h6-10H,1-5,11H2;1H/t6-,7+,8-,9+,10?;
InChI 密鑰
WLDWDRZITJEWRJ-ZDAMNCSYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
The HPLC assay of 2-adamantylamine hydrochloride after pre-column derivatization with 4-fluoro-7-nitro-2,1,3-benzoxadiazole has been studied.
應用
2-Adamantylamine hydrochloride was used to prepare 2-adamantylamide of 2′-(carboxymethoxime)-olivomycin I.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
C Clark et al.
Immunopharmacology, 21(1), 41-50 (1991-01-01)
The present in vitro investigations on amantadine (AmTd) and its isomer 2-aminoadamantane (2-NH2-Adam), and the corresponding analogs, 1-nitroadamantane (1-NO2-Adam) and 2-nitroadamantane (2-NO2-Adam), were undertaken to gain information about molecular features that might have a dominant role in inhibiting T lymphocyte
Yasuhiko Higashi et al.
Biomedical chromatography : BMC, 20(5), 423-428 (2005-09-15)
Simultaneous HPLC assay of 1-adamantanamine hydrochloride (amantadine) and its four related compounds [2-adamantanamine hydrochloride (2-ADA), 1-adamantanmethylamine (ADAMA), 1-(1-adamantyl)ethylamine hydrochloride (rimantadine) and 3,5-dimethyl-1-adamantanamine hydrochloride (memantine)] in phosphate-buffered saline (pH 7.4) after pre-column derivatization with 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F) was developed. Phosphate-buffered saline samples
Anna N Tevyashova et al.
The Journal of antibiotics, 62(1), 37-41 (2009-01-10)
A novel way of chemical modification of the antibiotic olivomycin I at the 2'-keto group of the side chain of the aglycone moiety was developed. Reaction of olivomycin I with the carboxymethoxylamine hemihydrochloride gave the key intermediate, 2'-carboxymethoxime-olivomycin I, which
S V Krapivin et al.
Biulleten' eksperimental'noi biologii i meditsiny, 116(11), 515-518 (1993-11-01)
The action of the new stimulant bromantane on spectra power EEG on Fourier of sensorimotor cortex, dorsal hippocamp and lateral hypothalamus of left and right hemispheres of brain of rat in free behavior was investigated. Bromantane leads to decreases in
Anastasiya V Lastovka et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 1132, 121813-121813 (2019-11-11)
The methods for quantification of highly potent analgesic agent (2R,4aR,7R,8aR)-4,7-dimethyl-2-(thiophen-2-yl)octahydro-2H-chromen-4-ol in rat whole blood and plasma were developed and validated using dried matrix spots (DMS) or fabric phase sorptive extraction (FPSE) techniques in combination with LC-MS/MS. 2-Adamantylamine hydrochloride was used
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務

![1-Bicyclo[1.1.1]pentylamine hydrochloride](/deepweb/assets/sigmaaldrich/product/structures/287/052/55f4f60a-a9e0-4ea2-b1e8-5b3f6ce0ff21/640/55f4f60a-a9e0-4ea2-b1e8-5b3f6ce0ff21.png)