推薦產品
品質等級
化驗
98%
形狀
powder
光學活性
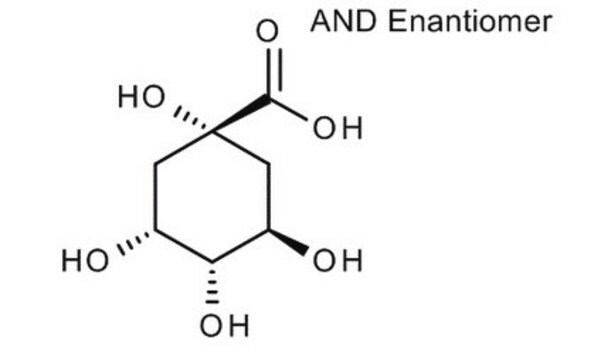
[α]20/D −43.9°, c = 11.2 in H2O
官能基
carboxylic acid
hydroxyl
SMILES 字串
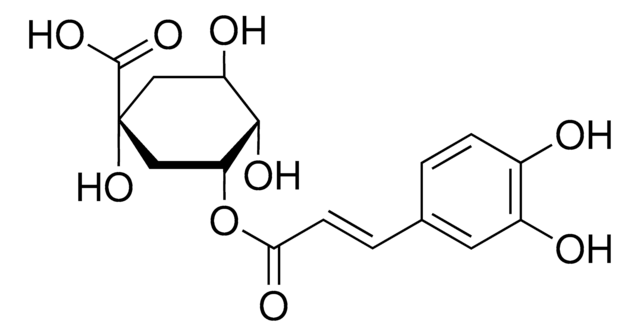
O[C@@H]1C[C@@](O)(C[C@@H](O)[C@H]1O)C(O)=O
InChI
1S/C7H12O6/c8-3-1-7(13,6(11)12)2-4(9)5(3)10/h3-5,8-10,13H,1-2H2,(H,11,12)/t3-,4-,5-,7+/m1/s1
InChI 密鑰
AAWZDTNXLSGCEK-WYWMIBKRSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
D -(-)-奎尼酸是一种植物代谢产物,是多步化学合成天然产物的手性结构单元。
應用
D -(-)-奎尼酸已被用作标准品,通过 HPLC 测定苦龙胆茶 和开发蔓越莓果实中有机酸的组成。可用于制备 3,4- O -异亚丙基-3 ( R ),4 ( S )-二羟基环己酮。
D-(−)-奎宁酸可:
- 与硫酸铜(II)一起用作手性选择电解质。该电解质用于通过配体交换毛细管电泳法对DL酒石酸进行手性拆分。
- 合成3,4,6-三羟基氮杂卓、7-羟甲基-3,4,5-三羟基氮杂卓和3,4,5-三羟基氮杂卓的立体异构体的起始物质,作为糖苷酶的潜在抑制剂。
- 用于制备三羟基哌啶衍生物和(+)-原栎醇糖苷酶抑制剂的前体。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Christopher J Potter et al.
Nature protocols, 6(8), 1105-1120 (2011-07-09)
In Drosophila, the GAL4/UAS/GAL80 repressible binary expression system is widely used to manipulate or mark tissues of interest. However, complex biological systems often require distinct transgenic manipulations of different cell populations. For this purpose, we recently developed the Q system
Tzenge-Lien Shih et al.
The Journal of organic chemistry, 72(11), 4258-4261 (2007-05-08)
Several new stereoisomers of 3,4,6-trihydroxyazepanes and 7-hydroxymethyl-3,4,5-trihydroxyazepanes as well as known 3,4,5-trihydroxyazepanes were synthesized as potent glycosidase inhibitors from D-(-)-quinic acid in an efficient manner. The key step employs dihydroxylation of protected chiral 1,4,5-cyclohex-2-enetriols under RuCl3/NaIO4/phosphate buffer (pH 7) condition
A unified asymmetric approach to substituted hexahydroazepine and 7-azabicyclo [2.2. 1] heptane ring systems from D (-)-quinic acid: Application to the formal synthesis of (-)-balanol and (-)-epibatidine
Albertini E, et al
Tetrahedron Letters, 38(4), 681-684 (1997)
d-(-)-Quinic acid: a chiron store for natural product synthesis
Barco A, et al
Tetrahedron Asymmetry, 8(21), 3515-3545 (1997)
Qiuling Li et al.
G3 (Bethesda, Md.), 6(10), 3351-3359 (2016-08-26)
Drosophila melanogaster is a powerful model organism for dissecting the molecular mechanisms that regulate sleep, and numerous studies in the fly have identified genes that impact sleep-wake cycles. Conditional genetic analysis is essential to distinguish the mechanisms by which these
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務