推薦產品
化驗
98%
形狀
liquid
折射率
n20/D 1.415 (lit.)
bp
119-121 °C (lit.)
mp
4.8 °C (lit.)
密度
0.812 g/mL at 25 °C (lit.)
官能基
hydroxyl
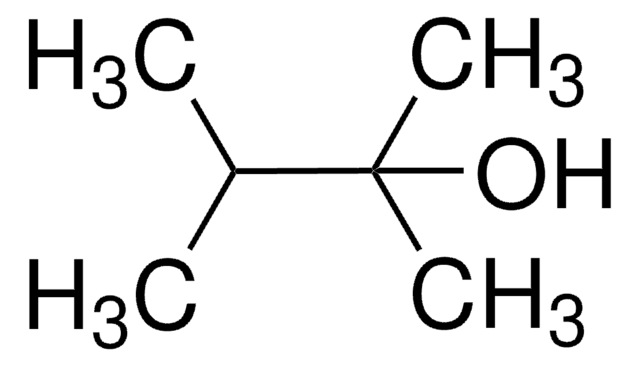
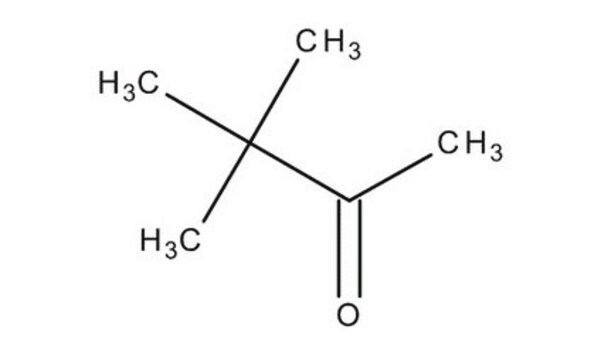
SMILES 字串
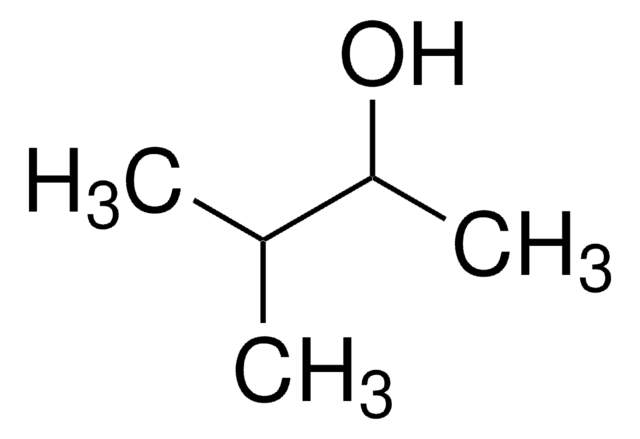
CC(O)C(C)(C)C
InChI
1S/C6H14O/c1-5(7)6(2,3)4/h5,7H,1-4H3
InChI 密鑰
DFOXKPDFWGNLJU-UHFFFAOYSA-N
一般說明
3,3-Dimethyl-2-butanol is a potential precursor for prohibited chemical weapons such as soman, a nerve agent. It is a synthetic analog of kairomone.
應用
3,3-Dimethyl-2-butanol (pinacolyl alcohol) can be used as a substrate:
- To study the oxidation of secondary alcohols to ketones using cyclic microwave heating technique.
- To prepare aryl ethers by reacting with aryl iodide using 4-pyrrolidinopyridine ligand via Cu-catalyzed Ullmann reaction.
3,3-Dimethyl-2-butanol was used in conversion of ribose- and glucose-binding proteins into receptors for pinacolyl methyl phosphonic acid.
訊號詞
Warning
危險聲明
危險分類
Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
78.8 °F - closed cup
閃點(°C)
26 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Chemical ionization mass spectral analysis of pinacolyl alcohol and development of derivatization method using p-tolyl isocyanate.
Murty MRVS, et al.
Analytical Methods : Advancing Methods and Applications, 2(10), 1599-1605 (2010)
Fast oxidation of secondary alcohols by the bromate-bromide system using cyclic microwave heating in acidic water
Paakkonen S, et al.
Tetrahedron Letters, 51(51), 6695-6699 (2010)
J T James et al.
Journal of applied toxicology : JAT, 7(5), 307-312 (1987-10-01)
Sprague-Dawley rats were given 15, 70 and 140 min exposures to 15 mg/l 3,3-dimethyl-2-butanol, pinacolyl alcohol (PA), or 6-hour exposures to 0.2, 1.0 and 5.0 mg/l PA (1 mg/l = 240 ppm). A 50% mortality rate was obtained at the
Ullmann CO coupling of sterically hindered secondary alcohols using excess amount of strongly coordinating monodentate ligands
Sugata H, et al.
Tetrahedron Letters, 58(10), 1015-1019 (2017)
W E Luttrell et al.
Biochemical pharmacology, 46(11), 2083-2092 (1993-12-03)
Soman (pinacolyl methylphosphonofluoridate), a highly toxic organophosphate compound, has been found to be a strong inhibitor of hepatic microsomal carboxylesterase in vitro, but an enhancer of carboxylesterase when administered in vivo. In response to this paradoxical observation, the objective of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務