推薦產品
品質等級
化驗
99%
mp
192-194 °C (lit.)
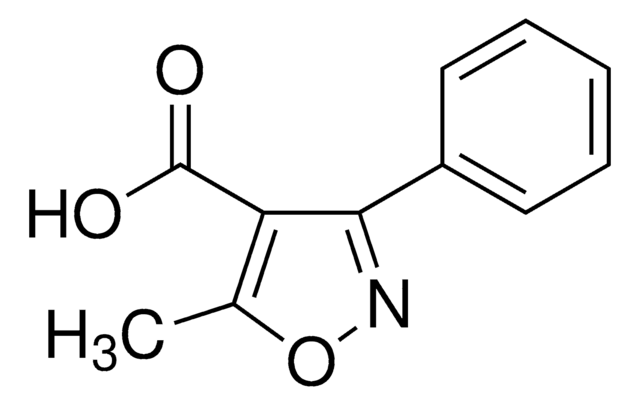
SMILES 字串
Cc1onc(-c2ccccc2)c1C(O)=O
InChI
1S/C11H9NO3/c1-7-9(11(13)14)10(12-15-7)8-5-3-2-4-6-8/h2-6H,1H3,(H,13,14)
InChI 密鑰
PENHKTNQUJMHIR-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
5-Methyl-3-phenylisoxazole-4-carboxylic acid was used in preparation of intermediates for the synthesis of penicillin. It was used for acylation during solid support synthesis of the isoxazolopyridone derivatives.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
1112. Derivatives of 6-aminopenicillanic acid. Part VI. Penicillins from 3-and 5-phenylisoxazole-4-carboxylic acids and their alkyl and halogen derivatives.
Doyle FP, et al.
Journal of the Chemical Society, 5838-5845 (1963)
Masayuki Nakamura et al.
Bioorganic & medicinal chemistry letters, 20(2), 726-729 (2009-12-17)
This Letter describes the synthesis and evaluation of mGluR7 antagonists in the isoxazolopyridone series. In the course of modification in this class, novel solid support synthesis of the isoxazolopyridone scaffold was developed. Subsequent chemical modification led to the identification of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務